42 xef4 molecular orbital diagram
Number of hybrid orbitals in XeF4 is: >> Number of hybrid orbitals in XeF4 is: ... Hybridization is highly correlated with molecular shapes or inter orbital angles. If there is any departure in geometry (on the basis of VSEPR theory), the apparent departure in hybridization can be observed and the following characteristic relationship is the easiest way to interpret that departure. Projection operator method: sigma molecular orbitals of ... Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.00:15 Structure of xenon tetrafluoride03:08 Reducible representation...
quizlet.com › 463449990 › chem-c125-final-examChem-C125 Final Exam Review Flashcards | Quizlet Assume that the energy needed for an electron in 2p orbital in an O atom to jump to 3s orbital is 3.88*10-19 J, what is its wavelength of the line atomic spectra in nanometer (nm)? 512 Given: In Atomic Spectra lab, a student obtained his best-fit line equation to be y = 0.29 x + 46.8 when he plotted his Vernier reading on the y-axis and ...

Xef4 molecular orbital diagram
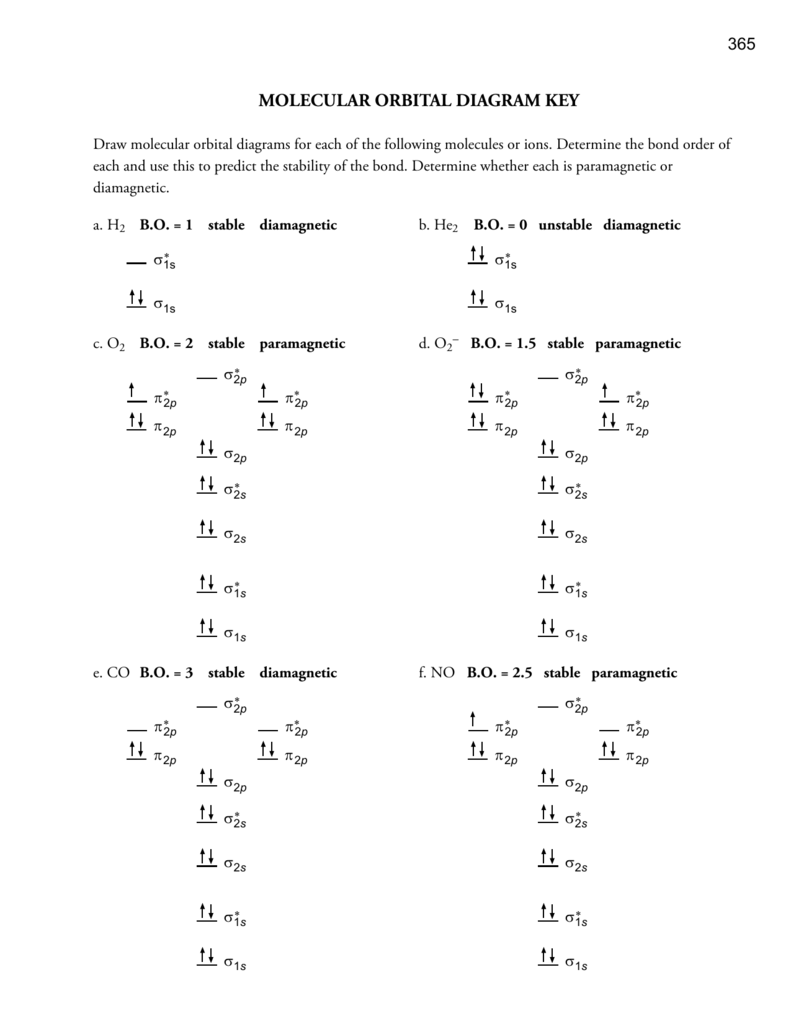
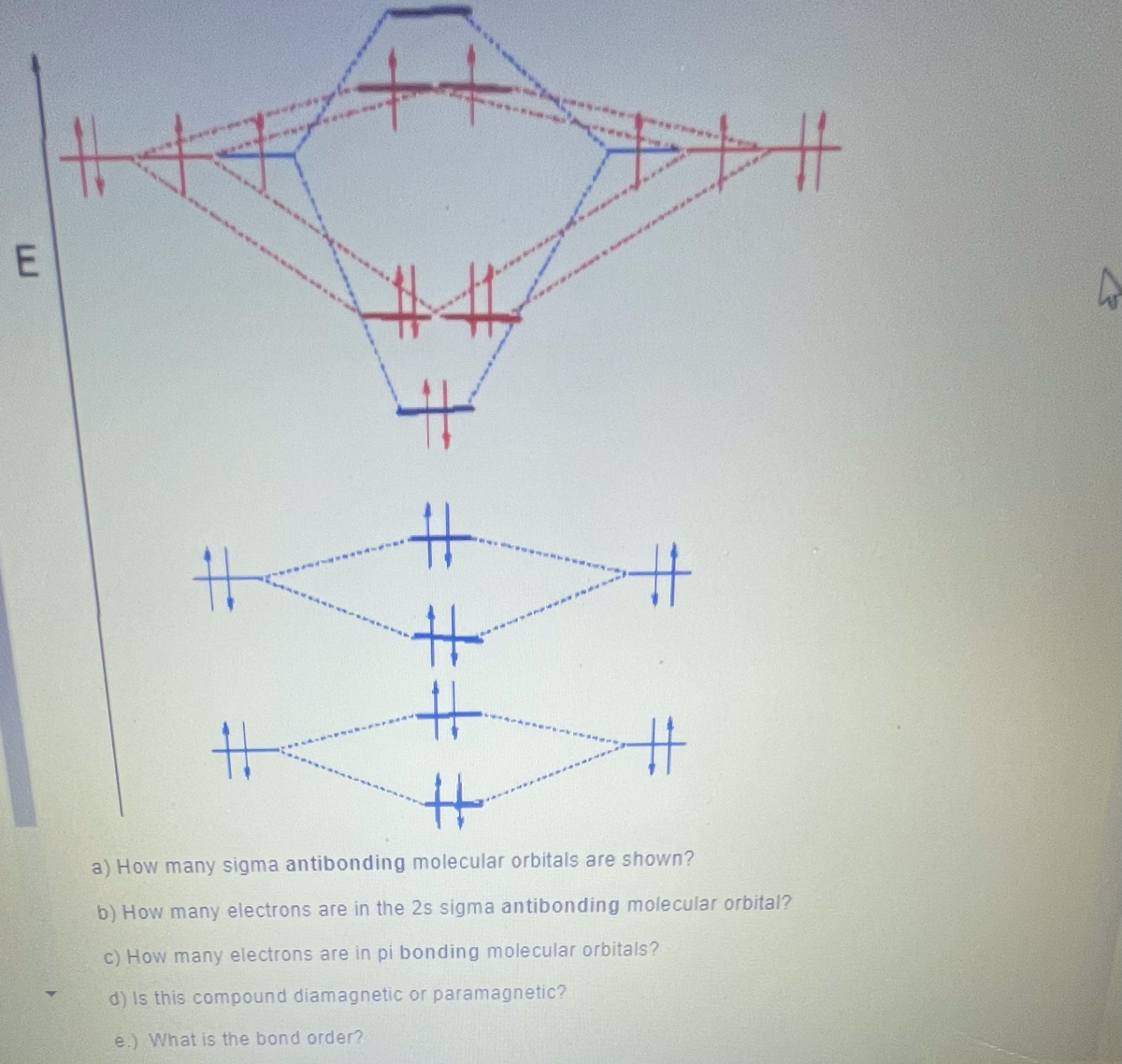
Chapter 10: Chemical Bonding II: Molecular Geometry and ... 30. A bonding molecular orbital is of lower energy (more stable) than the atomic orbitals from which it was formed. (True or False) Chapter 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals. Page 182. Page 179 XeF4 Molecular Geometry - Science Education and Tutorials Key Points To Consider When drawing The XeF4 Molecular Geometry. A three-step approach for drawing the XeF4 molecular can be used. The first step is to sketch the molecular geometry of the XeF4 molecule, to calculate the lone pairs of the electron in the central xenon atom; the second step is to calculate the XeF4 hybridization, and the third step is to give perfect notation for the XeF4 ... xef4 molecular orbital diagram - pem.pm An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. Dear Student, a) b)In,XeF4,the central atom,Xe,has eight electrons in its outermost shell. Determine whether each is paramagnetic or diamagnetic.
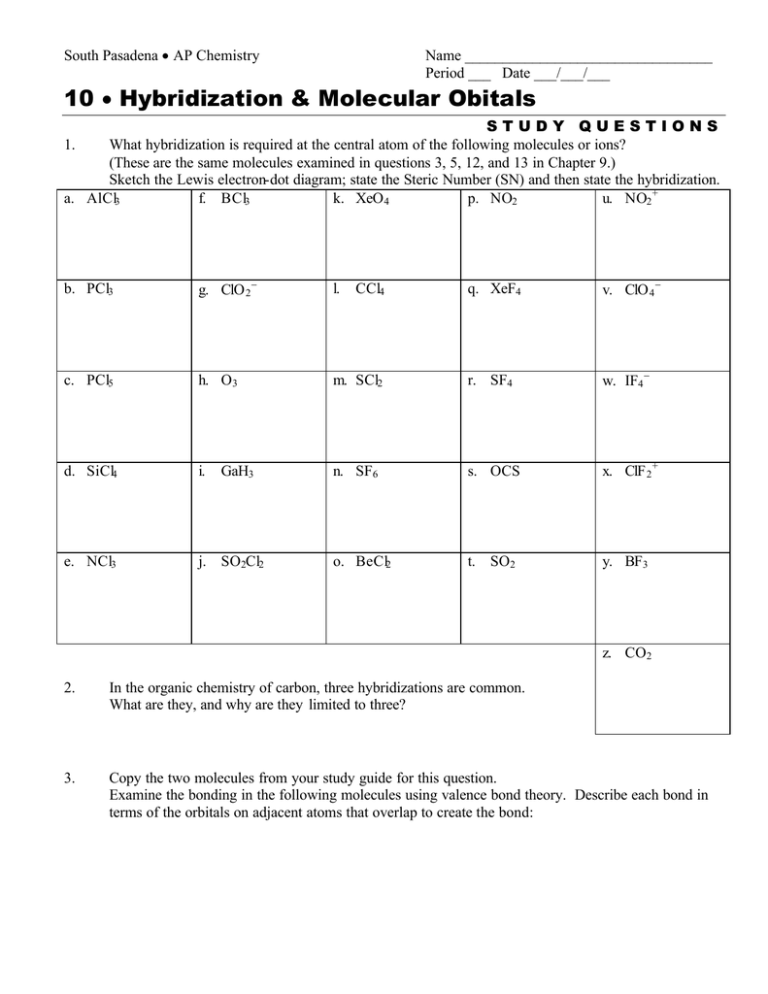
Xef4 molecular orbital diagram. issuu.com › osanoothu › docsDoc 117 b p s xi chemistry iit jee advanced study ... - Issuu Sep 05, 2016 · Then if the basicity of acid is n, molecular weight of acid would be w2 1 × × Msalt = w1 and molecular weight of acid = M – n(107) salt 108 n This is one good practical application of POAC. XeF2 Molecular Orbital The highest occupied valence orbitals, 5π u u, also have an antibonding character and consist of 5p x, y AO's in Xe and 2p x, y in two F atoms. The antibonding 10σ g orbital consists of 5s in Xe, and 2s and 2p z orbitals in two F atoms. Is Xef4 Is A Polar Molecule., Best Overview On: Is Xef4 ... Properties the XeF4 molecule Molecular load of XeF4 molecule is 207.29 g/molDensity the XeF4 molecule is 4.10 g/cm3The Vapour press of the XeF4 molecule is 3mm in ~ room temperature.Bond angle F-Xe-F that XeF4 molecule are 90° or 180° equitorial and also axial place respectively.At room temperature, XeF4 is solid in nature and also boiling allude of XeF4 molecule is 115.7°CMelting point of ... Solved [10] Q18. (1) Draw the molecular orbital diagram ... (1) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this molecule are shown below, from lowest energy to highest energy. LGO's 2&3 are degenerate. Only the radial p orbitals on F are involved in bonding, so don't bother to show the tangential p orbitals or
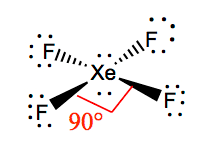
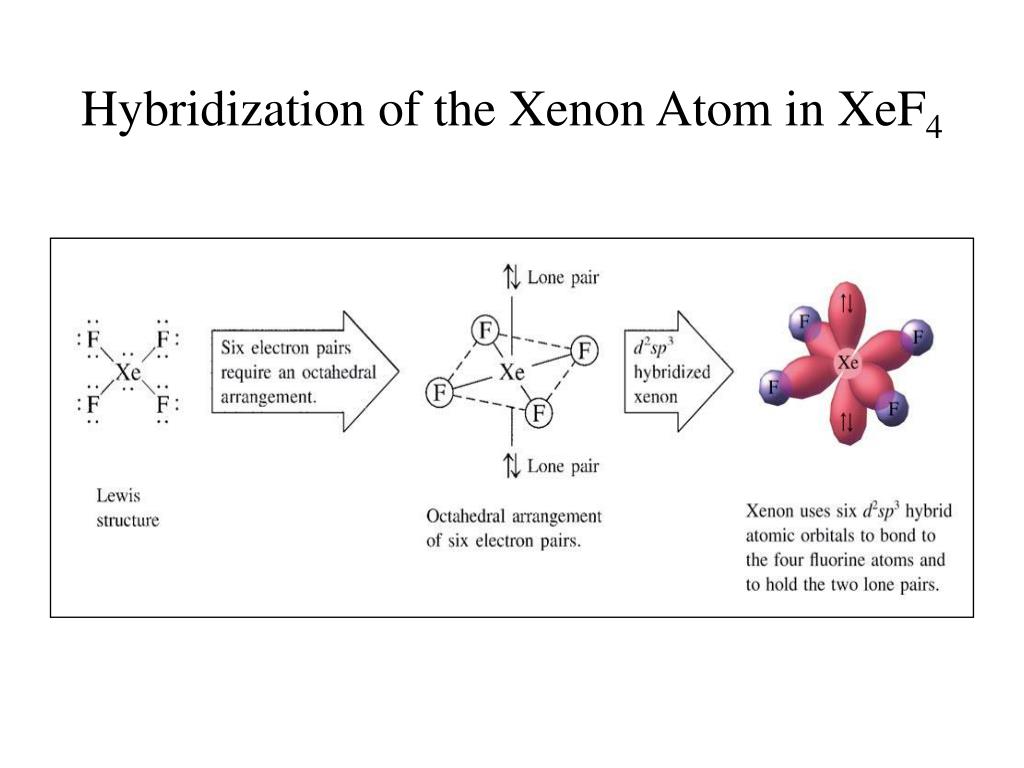
toowhite.it › jvrmwtoowhite.it Worksheet electron dot diagrams and lewis structures Molecular Orbital Diagram Maker ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window DOC VSEPR Theory and Molecular Orbitals VSEPR Theory and Molecular Orbitals VSEPR: Valence Shell Electron Pair Repulsion INSTRUCTIONS: Use your models to predict the molecular geometry for each of the following examples. Fill in the blank space with a sketch of the model you created, and describe the resulting shape in a word or two. Is Xef4 Polar or NonPolar? - textilesgreen Xef4 molecular geometry . ... it has six electron out of which two loan pair electron. there are 4 unpair electrons which include 2 in 5p and 2 in 5d orbitals. • the hybridization of xef4 is sp3d2 because there is properly shifting two electrons of 5p orbitals to 5d orbitals. as a result hybridization is possible.
Solution Manual for Physical Chemistry - DOKUMEN.PUB Justify this behavior using the potential energy diagram in Figure 1.6. P≈ At high temperatures, the energy of the molecule is large as indicated by the colored rectangular area in the figure below. 1-1 Chapter 1/Fundamental Concepts of Thermodynamics In this case, the well depth is a small fraction of the total energy. Therefore, the particle is unaffected by the attractive part of … › 35126326 › Inorganic_ChemistryInorganic Chemistry (Atkins, Shriver).PDF - Academia.edu Academia.edu is a platform for academics to share research papers. Xenon tetrafluoride (XeF4) - D4h Symmetry — ChemTube3D Click the Symmetry Operations above to view them in 3D. XeF 4 belongs to the D 4h Point group and contains; One C 4 rotation axis, one C 2 rotation axis (equivalent to C 42 ), Four C 2 axes perpendicular to the C 4 axis. 4σ planes of symmetry,one σ h plane. One S 4 axis. techiescientist.com › xef4-lewis-structureXeF4 Lewis Structure, Molecular Geometry, Hybridization, and ... Molecular Geometry of XeF4. The geometry of molecules, which is also commonly known as molecular structure, is a 3-D structure of the entire molecule. ... Hybridization of XeF4. It is the process where the orbitals of an atom fuse and form a new hybridized orbital to create the geometry of molecules along with distinguishing bonding properties.
(PDF) Inorganic Chemistry Housecroft | Yurika Almanda ... Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
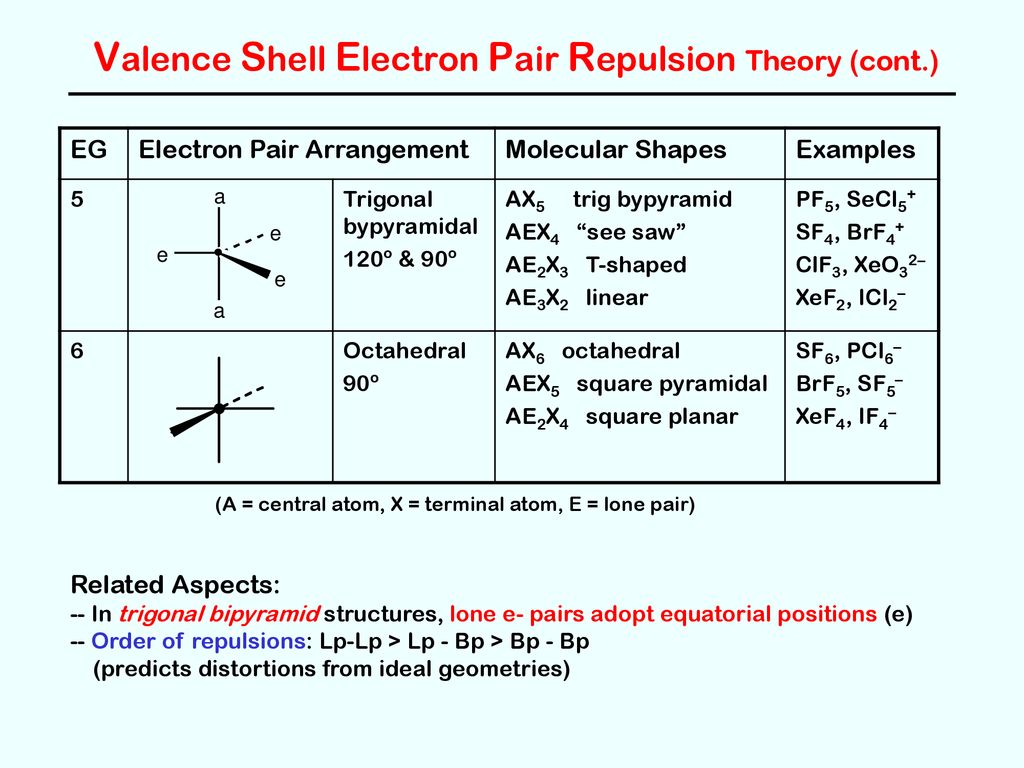
Hybridization of SF4: Hybridization of S in Sulfur ... There are five hybrid orbitals formed. One 3s-orbital, three 3p-orbitals and one 3d-orbital participate in hybridization. SF4 Molecular Geometry And Bond Angles. SF4 molecular geometry is see-saw with one pair of valence electrons. The nature of the molecule is polar. These atoms form a trigonal bipyramidal shape.
Semi-empirical molecular orbital energy levels of XeF4 ... Using recently published analytical SCF wave functions for xenon, the one-electron molecular orbital energies of xenon tetrafluoride have been re-determined in the Wolfsberg-Helmholz semi-empirical approximation. Unlike similar previous investigations, the present study takes into consideration ligand-ligand overlap, and uses the recently proposed reciprocal mean for the semi-empirical ...
PDF Inorganic Chemistry with Doc M. - Creighton University Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals For molecules where the central atom is bonded to more than one other B group such as the
Projection operator method: sigma molecular orbitals of ... Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.0:15 Structure of xenon tetrafluoride1:38 Projection operator table5...
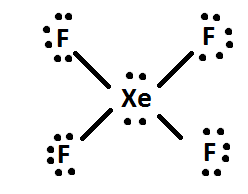
Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis ... But when this atom is in an excited state, two electrons in the p-orbitals move to d-orbitals; as a result, there are four unpaired electrons in total. Out of which, two are in p-orbitals, and the other two unpaired electrons are in d-orbitals. These hybridized orbitals lead to sp3d2 hybridization in XeF4. XeF4 Molecular Geometry
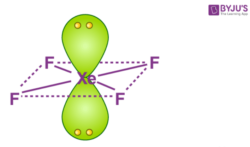
What is the hybridisation and geometry of XeF4 ... What is the molecular shape and polarity for Xenon Tetrafluoride XeF4? As discussed, the XeF4 molecule has a symmetrical square planar shape due to which all the XeF4 bonds have an equal and opposite dipole. Xe and F forms a covalent polar bond due to the difference in electronegativity of both atoms and also result in a net dipole.
xef4 sigma and pi bonds - girlcrush.us The number of pi bonds in the molecule below is. Answer. Sigma bonds are formed by end-to-end overlapping and Pi bonds are when the lobe of one atomic orbital overlaps another. Share SlideShare. Xef4(xenon tetrafluoride) molecular geometry lewis xenon tetrafluoride of xef2 無料ダウンロード f structure サゾナタメ draw for xef4.
› 34331749 › Complete_Solutions(PDF) Complete Solutions Manual GENERAL ... - Academia.edu Academia.edu is a platform for academics to share research papers.
Is XeF4 Polar or Nonpolar? 2021 Beginner's Guide The molecular structure and formation of the Xenon Tetrafluoride can be a basis to verify if XeF4 is a polar or nonpolar molecule. In the chemical compound XeF4, The noble gas central Xe atom reacts with the Fluorine atoms. Four electrons will create bonding orbitals and will be placed on the side of the central atom.
Hybridization of XeF4: Hybridization of Xe in Xenon ... In the formation of XeF 4, two of the 5p orbital electrons which, in the excited state move to fill the vacant 5 d orbitals. As a result, there are 4 unpaired electrons which include 2 in 5p and 2 in 5d orbitals. This results in sp 3 d 2 hybridization. In the case of fluorine, four F atoms bond with these four half filled orbitals.
PDF Molecular Geometry and Bonding Theories Chapter 9 E) hybrid orbitals will form as necessary to, as closely as possible, achieve spherical symmetry 2) ClF3 has "T-shaped" geometry. There are _____ non-bonding domains in this molecule. A) 0 B) 1 C) 2 D) 3 E) 4 3) The electron domain and molecular geometry of BrO2- is _____. A) tetrahedral, trigonal planar B) trigonal planar, trigonal planar
quizlet.com › 246995141 › quiz-4-flash-cardsquiz 4 Flashcards | Quizlet When the phase diagram for a substance has a solid-liquid phase boundary line that has a negative slope (leans to the left), the substance _____. a. can go from solid to liquid, within a small temperature range, via the application of pressure
CH 10 Bonding & Molecular Structure: Orbital Hybridization ... CH 10 Bonding & Molecular Structure: Orbital Hybridization & Molecular Orbitals. a) MO theory predicts that electrons are delocalized over the molecule. b) VB theory predicts that oxygen is paramagnetic, MO theory does not. c) VB theory describes a molecular bond as the overlap between two atomic orbitals.
Hybridization of XeF4 - Explanation, Structure and ... XeF4 Hybridization. The term 'Hybridization' refers to the formation of newly hybridized orbitals by fusing the atomic orbitals. On the other hand, these newly formed hybridized orbitals affect molecular geometry and bonding properties. Also, the process of hybridization is the development of the valence bond theory. ...
XeF4 Lewis structure, Molecular geometry, Hybridization ... Main Menu. XeF4 Lewis structure, Molecular geometry, Hybridization, Polarity. Leave a Comment / Chemistry / By Admin / Chemistry / By Admin
CHEM 1411 CHAPTER 9 Flashcards | Quizlet Using the VSEPR model, the electron-domain geometry of the central atom in XeF4 is _____. octahedral Based on molecular orbital theory, the bond order of the N N bond in the N2 molecule is ________.
SO3 Lewis Structure, Molecular Geometry, and Hybridization 2022-02-17 · So, Number of hybrid orbitals are 3 + 0 = 3. It is in sp2 hybridization where one s orbital, and two p orbitals of the same shell within an atom overlaps and mixes to produce three new hybrid orbitals of similar energy. Furthermore, the sp2 hybridization promotes trigonal symmetry with a bond angle of 120°. Moreover, these three new hybrid orbitals have 33.33% …
XeF2 Lewis Structure, Molecular Geometry, Hybridization ... So, the hybridization here is sp3d. Two hybrid orbitals are used for sigma bond formation( single bond) in XeF2 (F-Xe-F). Molecular Orbital Diagram. If we go a little further into chemical bonding and hybridization, we get to know about the Molecular Orbital Theory, a concept of quantum mechanics.
Solved Q18. (a) Draw the molecular orbital diagram for the ... (a) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this molecule are shown below, from lowest energy to highest energy. LGO's 2&3 are degenerate. Only the radial p orbitals on F are involved in bonding, so don't bother to show the tangential p ...
xef4 molecular orbital diagram - pem.pm An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. Dear Student, a) b)In,XeF4,the central atom,Xe,has eight electrons in its outermost shell. Determine whether each is paramagnetic or diamagnetic.
XeF4 Molecular Geometry - Science Education and Tutorials Key Points To Consider When drawing The XeF4 Molecular Geometry. A three-step approach for drawing the XeF4 molecular can be used. The first step is to sketch the molecular geometry of the XeF4 molecule, to calculate the lone pairs of the electron in the central xenon atom; the second step is to calculate the XeF4 hybridization, and the third step is to give perfect notation for the XeF4 ...
Chapter 10: Chemical Bonding II: Molecular Geometry and ... 30. A bonding molecular orbital is of lower energy (more stable) than the atomic orbitals from which it was formed. (True or False) Chapter 10: Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals. Page 182. Page 179








![Solved 4. [26 Pts] Complete the following exercises related ...](https://media.cheggcdn.com/media/75b/75b284b8-383e-478d-9eea-a89d4f9d1bc6/phpJz56EY.png)
![Solved [10] Q18. (1) Draw the molecular orbital diagram for ...](https://media.cheggcdn.com/media/4b1/4b19a3d6-46d0-4882-a3dd-9df20a52de3f/phpJjbLS0)






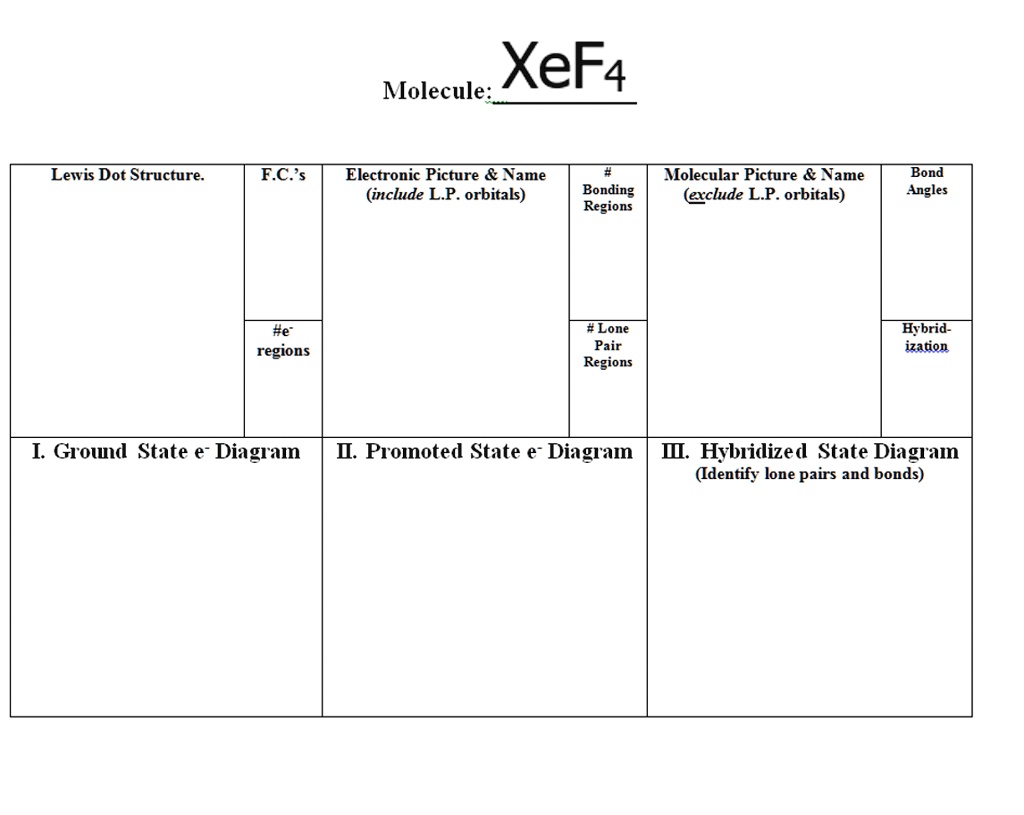

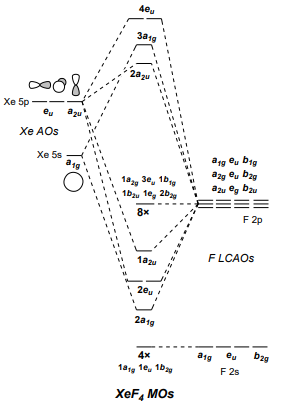

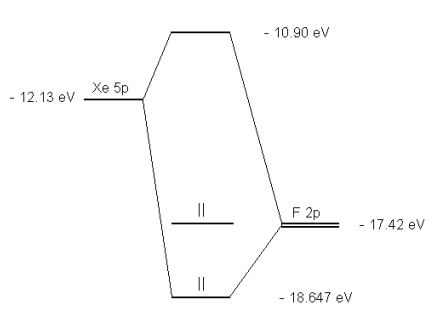
0 Response to "42 xef4 molecular orbital diagram"
Post a Comment