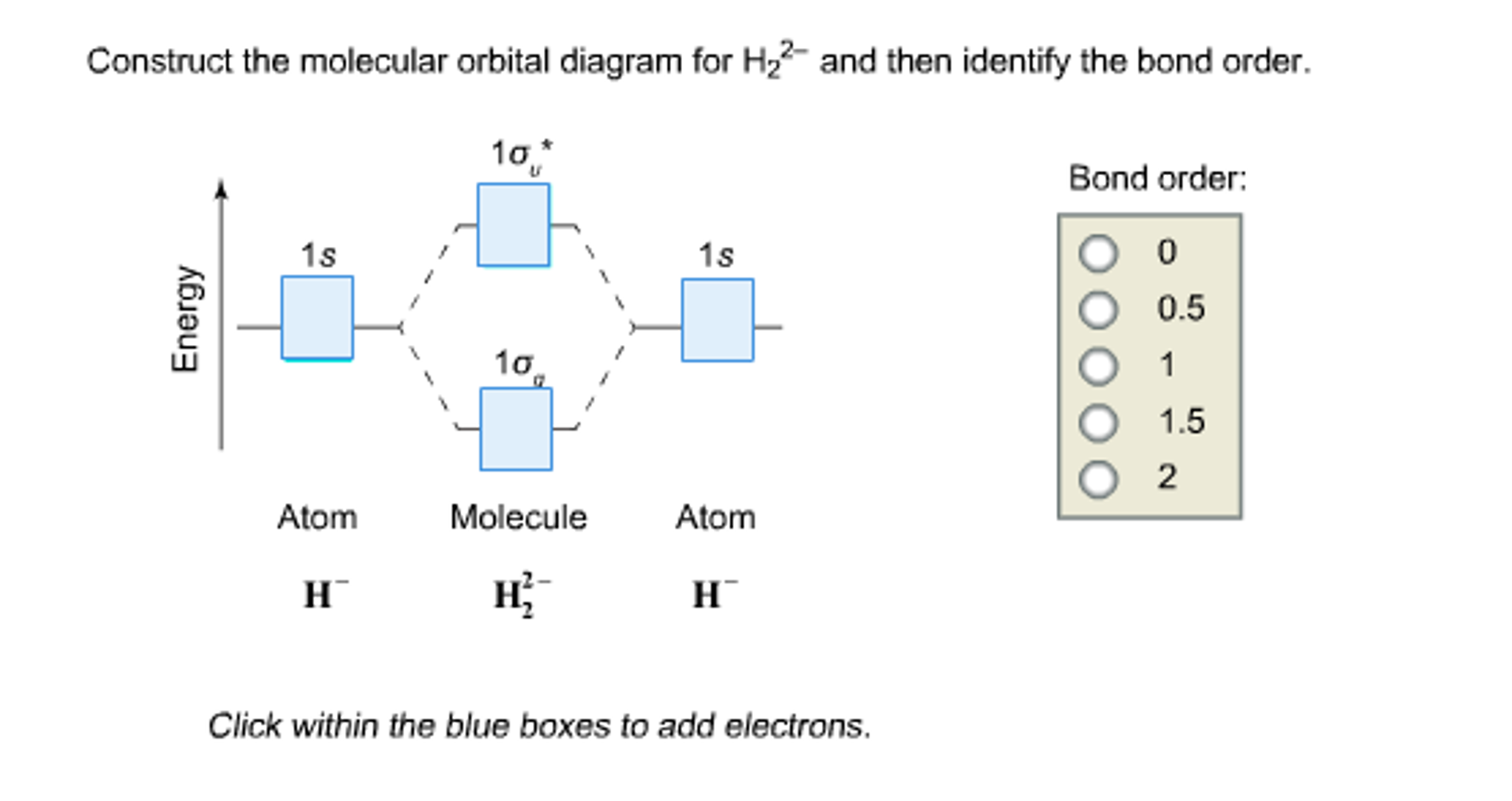
38 construct the molecular orbital diagram for h2 and then identify the bond order.
(PDF) General chemistry 5th edition | MOHD ... - Academia.edu (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Construct the molecular orbital diagram for H2 and then ... Construct the molecular orbital diagram for H2 and then identify the bond order. Click within the blue boxes to add electrons. A solution of H2SO4(aq) with a molal concentration of 2.24 m has a density of 1.135 g/mL. What is the molar concentration...
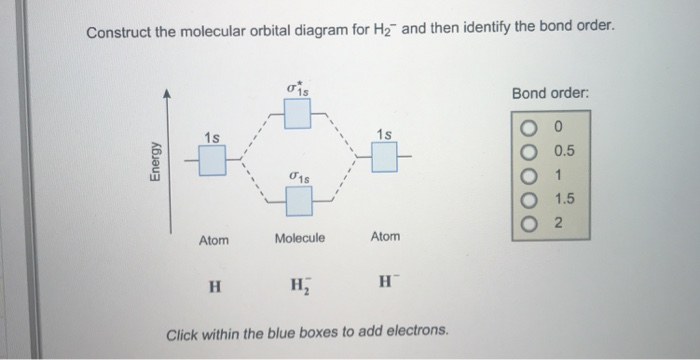
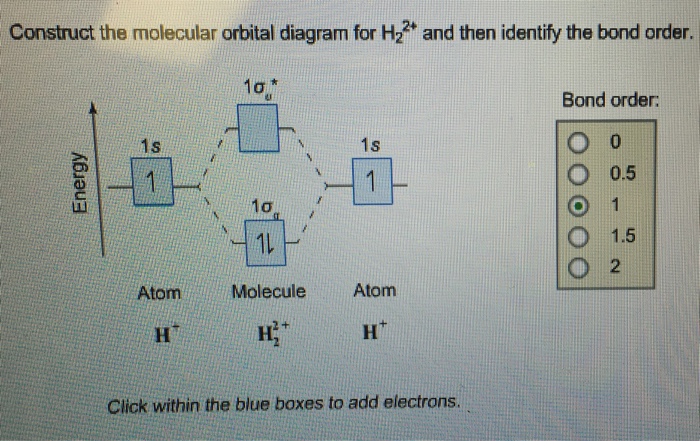
Construct the molecular orbital diagram fo... | Clutch Prep Problem: Construct the molecular orbital diagram for H2- and then identify the bond order. Click thin the blue boxes to add electrons.Bond order: a) 0 b) 0.5c) 1 d) 1.5e) 2

Construct the molecular orbital diagram for h2 and then identify the bond order.
Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ... Construct The Molecular Orbital Diagram For He2 And Then ... Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order. Calculate the bond order by subtracting the number of antibonding electrons from bonding electrons and then dividing the difference by two. 4. Assign the para just now. servantes. Molecular Orbital Diagram For He2. PDF Polyatomic Molecular Orbital Theory - La Salle University Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
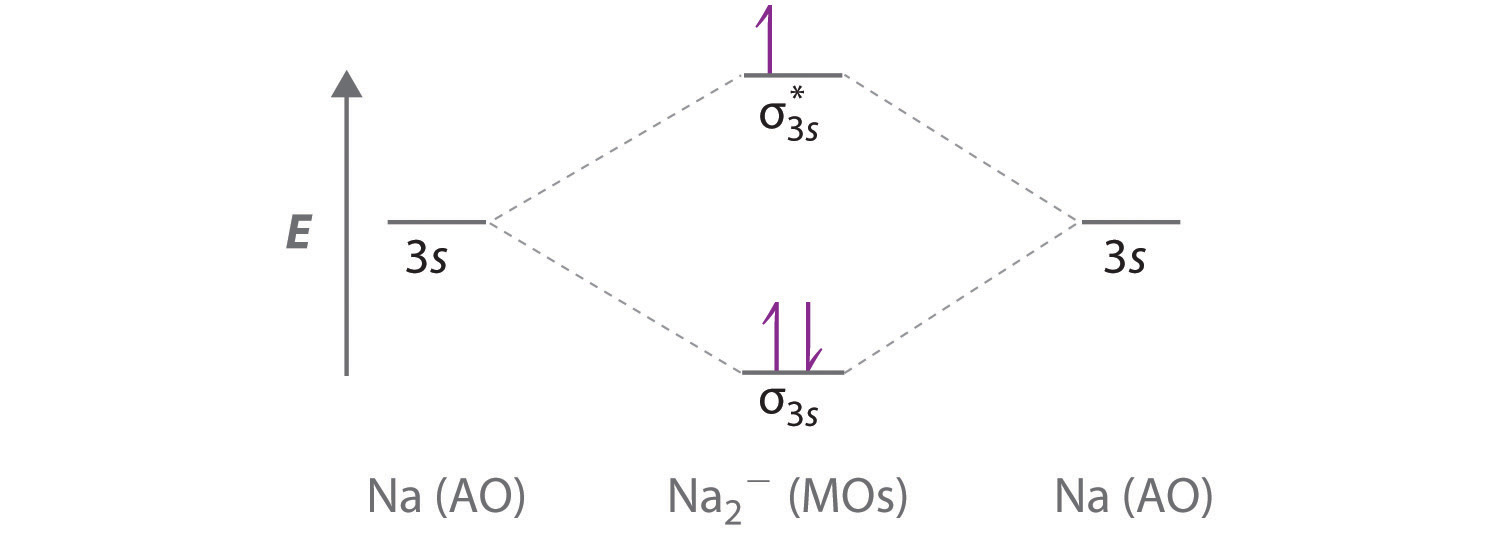
Construct the molecular orbital diagram for h2 and then identify the bond order.. Construct The Molecular Orbital Diagram For He2 And Then ... Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. Since both molecular ions have a bond order of 1/2, they are approximately equally stable. Problem: Surprisingly, the hybridization of the starred oxygen in the following molecule is sp 2, not sp 3. Construct the molecular orbital diagram fo... | Clutch Prep Construct the molecular orbital diagram for He 2 and then identify the bond order. Click within the blue boxes to add electrons. Bond order: a) 0. b) 0.5. c) 1. d) 1.5. e) 2. Learn this topic by watching MO Theory: Bond Order Concept Videos. Solved Construct the molecular orbital diagram for H2- and ... View the full answer. Transcribed image text: Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: 0 0.5 1 1.5 2 Click within the blue boxes to add electrons. Previous question Next question. Construct the molecular orbital diagram for H2+ and then ... Construct the molecular orbital diagram for H2- and then identify the bond order. Construct the molecular orbital diagram for H2- and then identify the bond order.Bond order:00.511.52 Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click...
Solved Construct the molecular orbital diagram for H2- and ... Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Construct The Molecular Orbital Diagram For He2 And Then ... Bond order: Click within the blue boxes to add electrons. Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. Show transcribed image text Construct the molecular orbital diagram for He2 and then identify the bond order. 1s Bond order: 、、 1s 0 O O 2 Atom. Construct The Molecular Orbital Diagram For H2 And Then ... Construct the molecular orbit diagram for H2- and then identify the bond order. Shortcut order: Click slim the blue box to include electrons. The concept used to settle this problem is based on molecular orbit diagram. Construct the molecular orbital diagram for h2- and then ... ChemistryHelper2024. Since 1s shell of bonding orbital can accommodate only two electrons. So, next one electron will go into 1 s shell of anti-bonding orbital. - Bond order = (Bonding electrons - antibonding electrons) / 2. = (2 - 1) / 2 = 0.5. search. rotate. apsiganocj and 20 more users found this answer helpful. heart outlined.
Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . 40 molecular orbital diagram for he2+ - Diagram For You Molecular Orbital Diagram Of H2 - schemacheck.com Molecular Orbital Diagram Of H2 For the diatomic molecules like hydrogen and helium, the following molecular orbital diagram is used. antibonding molecular orbital Energy - Is. For H2, bond order = 1/2 = 1, which means H2has only one bond. The antibonding orbital is empty. OneClass: construct the molecular orbital diagram for He2 ... Construct the molecular orbital diagram for H2 and then identify the bond order. Make sure you add electrons to the boxes corresponding to the MOs for the molecule and to the boxes corresponding to the AOs for the two atomic species. Bond order: 1s o 0.5 O 1.5 2 Atom Molecule Atom Click within the blue boxes to add electrons. OneClass: Construct the molecular orbital diagram for H2 ... Construct the molecular orbital diagram for H2 and then identify the bond order. Make sure you add electrons to the boxes corresponding to the MOs for the molecule and to the boxes corresponding to the AOs for the two atomic species. Bond order: 1s o 0.5 O 1.5 2 Atom Molecule Atom Click within the blue boxes to add electrons.
Draw molecular orbital diagram of O2 or N2 with magnetic ... As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [ N b − N a ] / 2 = [ 1 0 − 6 ] / 2 = 2 .
Fundamentals Of Thermodynamics 8th edition - Academia.edu Academia.edu is a platform for academics to share research papers.
Answered: construct the molecular orbital diagram… | bartleby Solution for construct the molecular orbital diagram for H2− and then identify the bond order. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. ...
Molecular orbital diagram of h2 - fornoob.com Molecular orbital diagram of hydrogen molecule: Bond order: From the molecular orbital diagram, there are 2 electrons in bonding molecular orbital and there is no anti - bonding molecular orbital. The bond order can be determined by substituting those values using bond order formula. Take care while calculating the bond order.
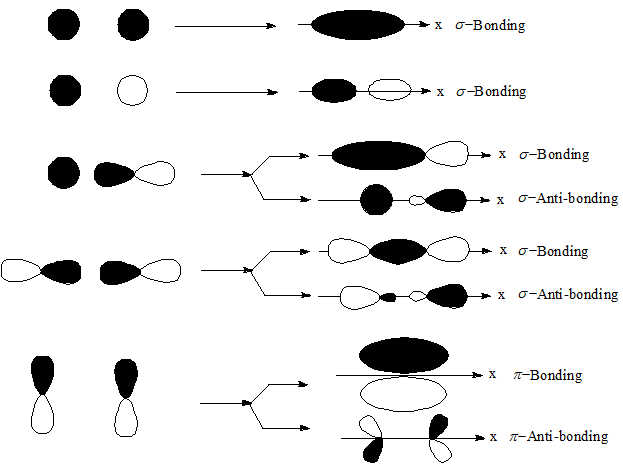
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
8.4 Molecular Orbital Theory - Chemistry A dihydrogen molecule contains two bonding electrons and no antibonding electrons so we have. bond order in H2 = (2−0) 2 = 1 bond order in H 2 = ( 2 − 0) 2 = 1. Because the bond order for the H-H bond is equal to 1, the bond is a single bond. A helium atom has two electrons, both of which are in its 1 s orbital.
oxygen fluoride molecular orbital diagram landmarks in guatemala city oxygen fluoride molecular orbital diagram. Posted on February 21, 2022 by February 21, 2022 by
(Get Answer) - Cation Anion Formula Name Magnesium ... Cation Anion Formula Name Magnesium bicarbonate SrCl2 Selection A Manganese II) chlorate 2+ Co PO4 3- Selection B Cu2CO3 Selection C Construct the molecular orbital diagram for N2 and then identify the bond order Bond order 0.5 O 1.5 O 2.5 2s 2s Click within the blue boxes to add electrons.
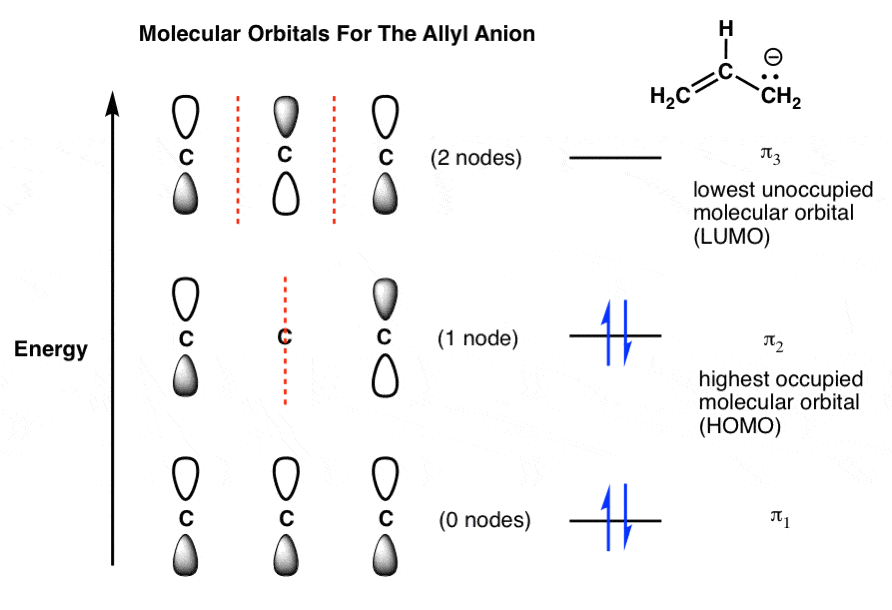
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital Similar phase of electron density (no node) adds together constructively. energy of isolated atoms bond order (H2 molecule) = (2) - (0) 2 = 1 bond 1sb H H H H σ∗ = 1s H H a - 1sb = antibonding MO = LCAO = linear combination of atomic orbitals node = zero ...
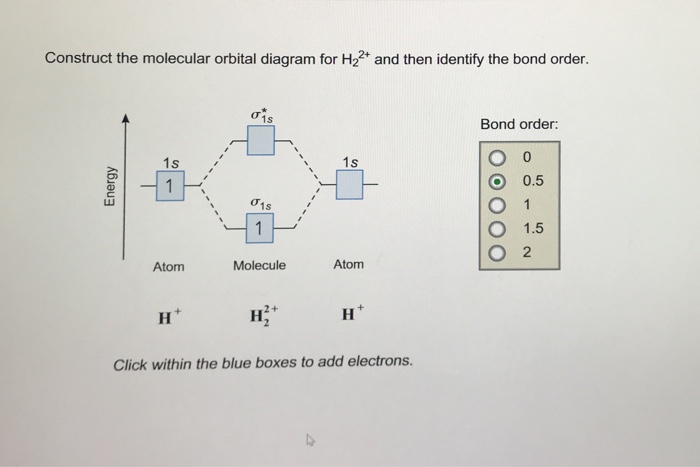
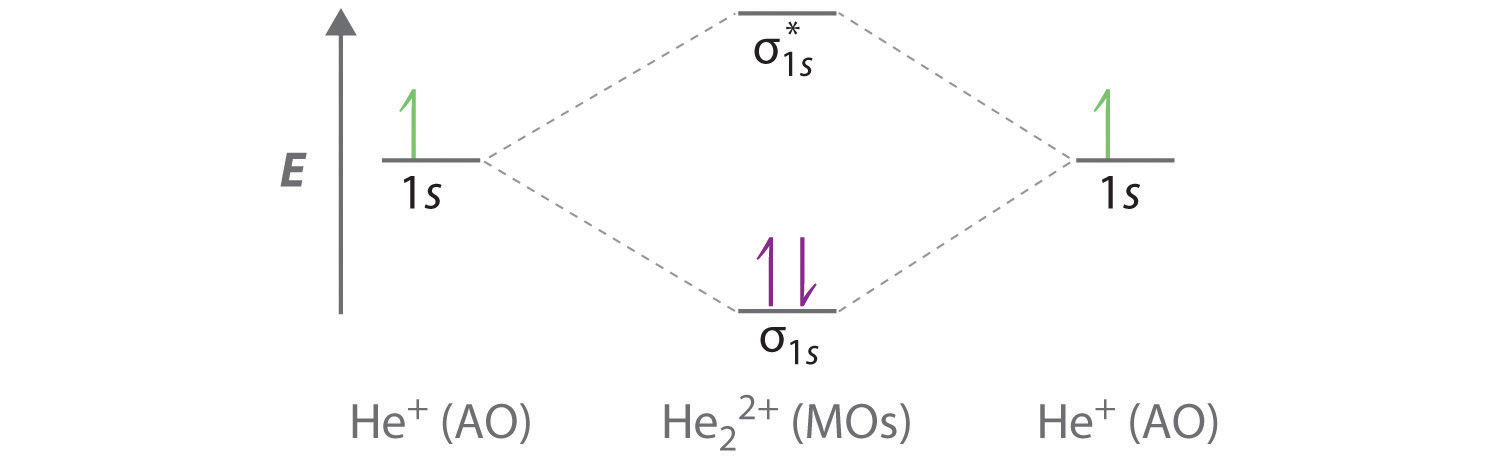
He2 2+ Molecular Orbital Diagram - Wiring Diagrams The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe.
Construct the molecular orbital diagram for H2- and then ... Complete this molecular orbital diagram for CN- then determine the bond order. Note that the 1s orbital is not shown in this problem. To add arrows to the MO diagram, click on the blue boxes.Bond order of CN-00.511.522.53. Construct the molecular orbital diagram for He.
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
PDF Polyatomic Molecular Orbital Theory - La Salle University Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
Construct The Molecular Orbital Diagram For He2 And Then ... Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order. Calculate the bond order by subtracting the number of antibonding electrons from bonding electrons and then dividing the difference by two. 4. Assign the para just now. servantes. Molecular Orbital Diagram For He2.
Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ...






















0 Response to "38 construct the molecular orbital diagram for h2 and then identify the bond order."
Post a Comment