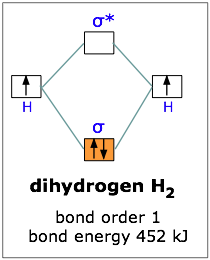
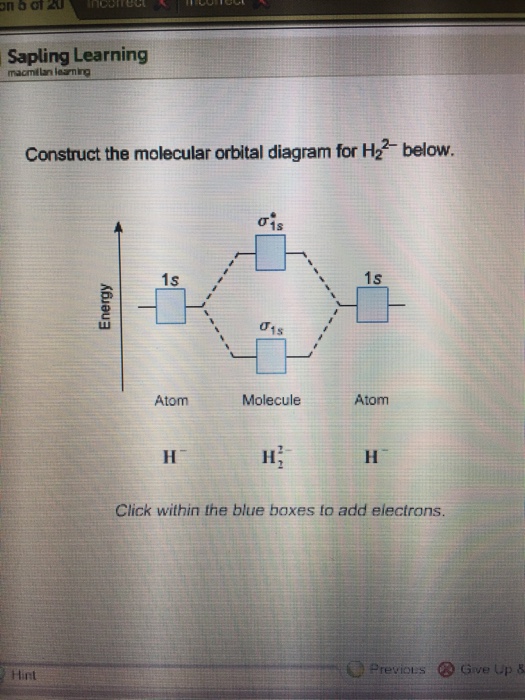
42 construct the molecular orbital diagram for h2 below
So, no need to placing electrons around Given below is the molecular orbital diagram for CH4. 2 – is. The electronic configuration of carbon (Z = 6) in the excited state is. Q:- The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. 1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is .
0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo...

Construct the molecular orbital diagram for h2 below
Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. 1s2, 2s2, 2p6, 3s2 ... Determine the molecular geometry of N2O (oxygen is terminal). trigonal pyramidal bent tetrahedral trigonal planar linear. ... Which molecule below … To write the dot formula, construct the molecular model, and determine the molecular shape and polarity (dipole or not) for each of the following molecules or polyatomic ions. Note, with the except... In this case, the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory – . Item 2: Part A Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below.M.O. diagram for N2+Molecular orbital diagram - Wikipedia
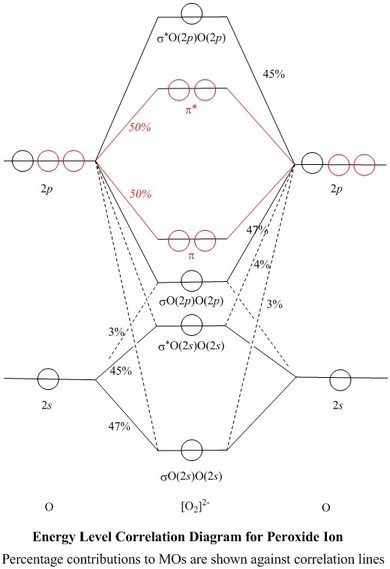
Construct the molecular orbital diagram for h2 below. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. 1) nitrogen triiodide (NI 3 Electron Orbital Activity (50pts) Background Information: The arrangement of electrons within the orbitals of an atom ... Molecular orbital diagram for ne2 2 . Molecular orbital diagram for ne2 2 Molecular orbital diagram for ne2 2 ... Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a hero nuclear diatomic molecule, determine bond order for each below. Oxygen – O 2. This molecular Look at the MO diagrams of corresponding neutral diatomic species in Figure 7. Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
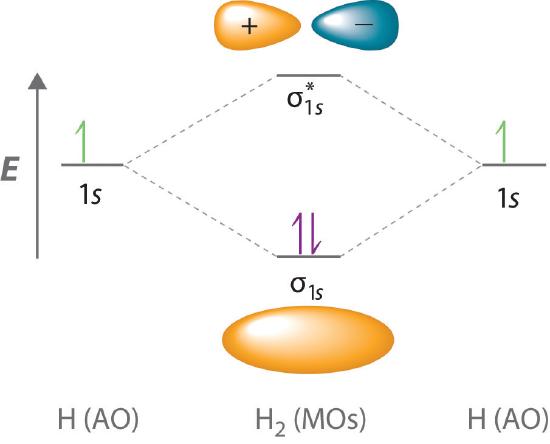
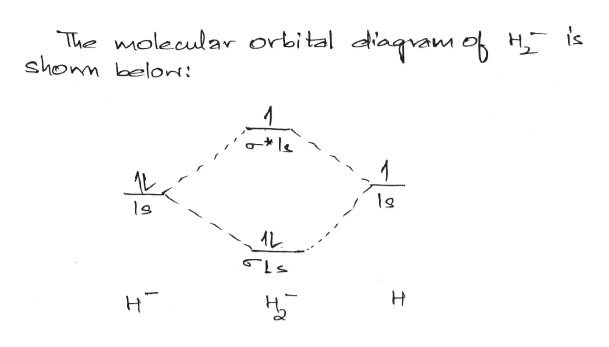
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. Mar 04, 2021 · Describe the essential difference between a sigma and a pi molecular orbital. Define bond order, and state its significance. Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. Nov 16, 2021 · Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. How does the number of electron . September 15, 2021 on Lewis Electron Dot Diagram Worksheet Answers. This is illustrated by the drawings below. CCl 2 F 2 d. Electron Configuration Orbital Diagram Worksheet Answers 21 hours ago · The five steps. Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
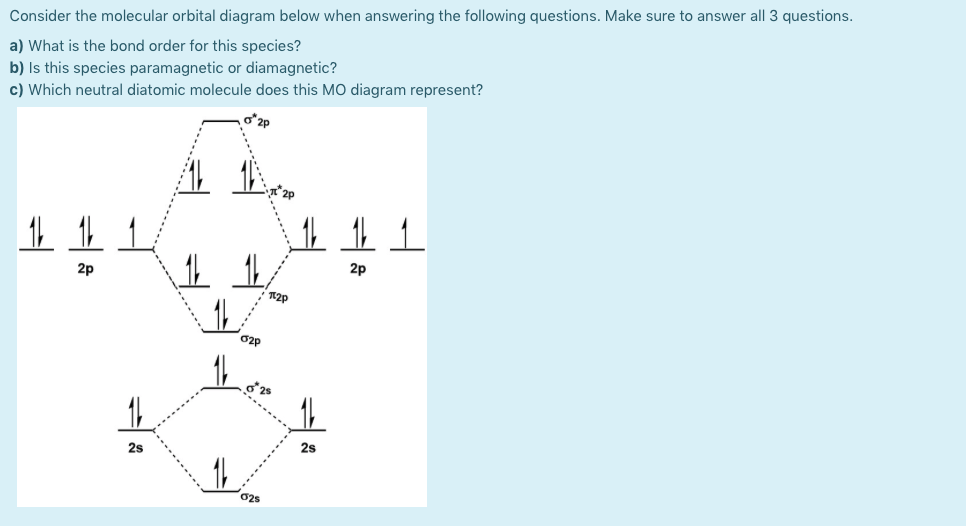
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic B2 Place the following in order of decreasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in each molecule. Discussed in this video are. Draw mo energy diagrams for the molecular ions h2 and h2. Construct the molecular orbital diagram for h2. When the 1s wave functions of the two ceh atoms are linearly combined we get a sigma s bonding orbital denoted as s 1s in the diagram herethis approach is called linear combination of atomic orbitals lcao. Science. Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order.
Academia.edu is a platform for academics to share research papers.
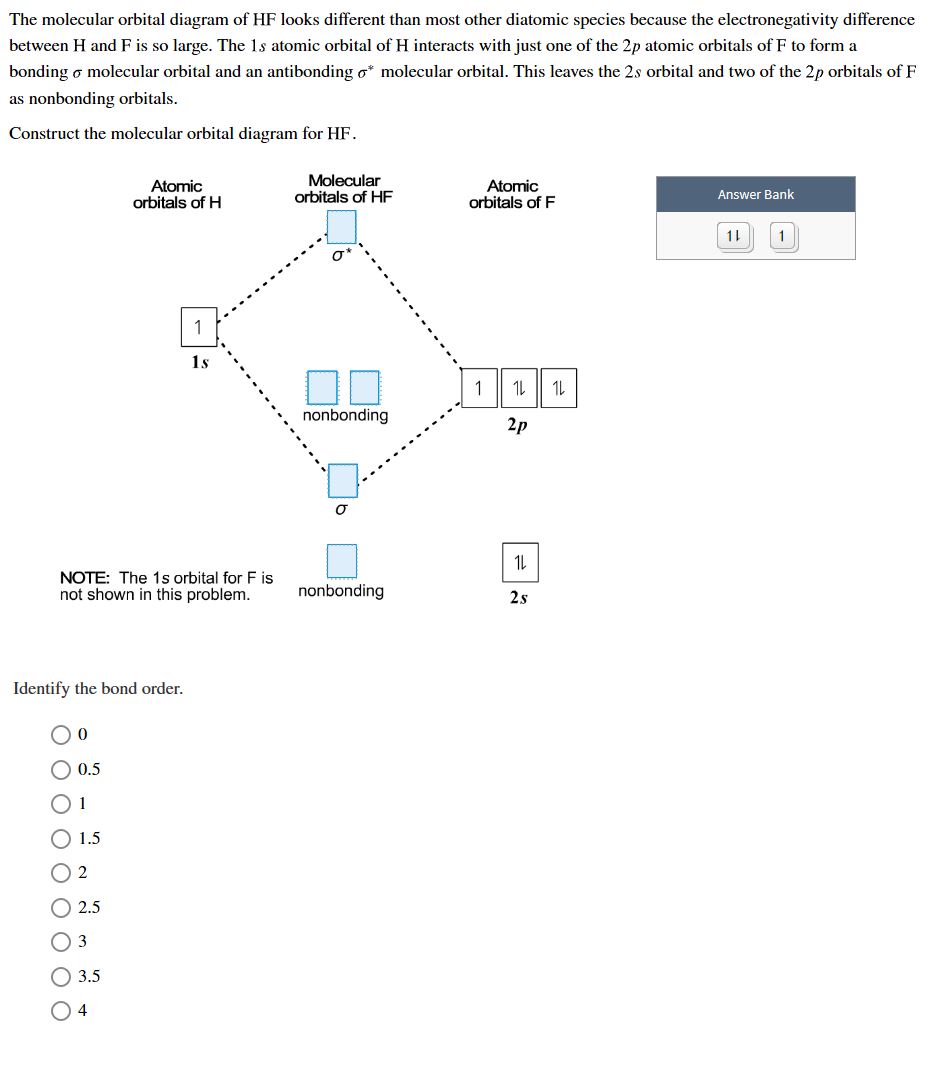
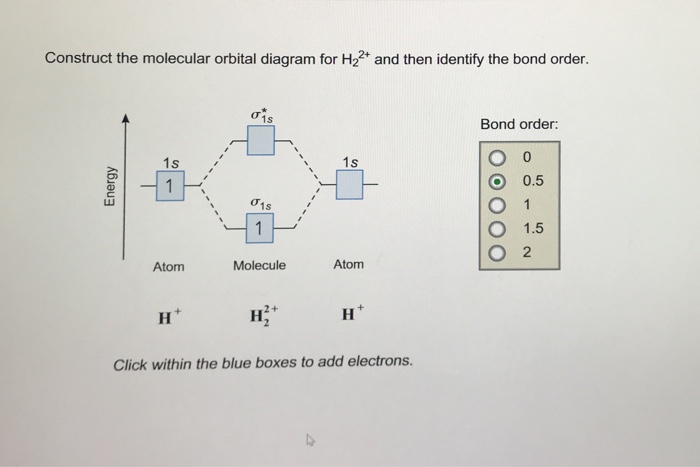
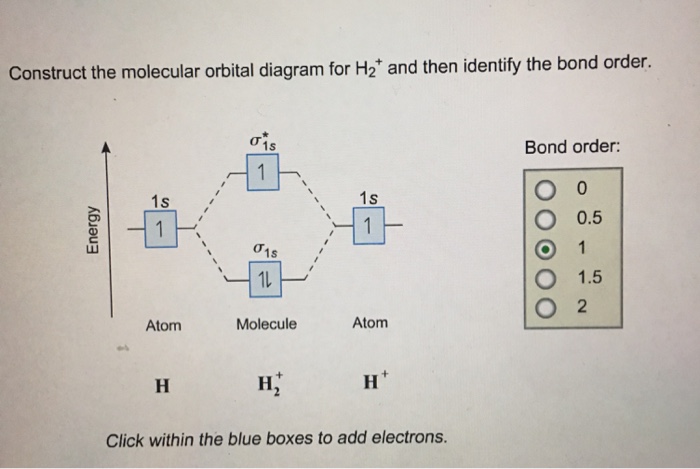
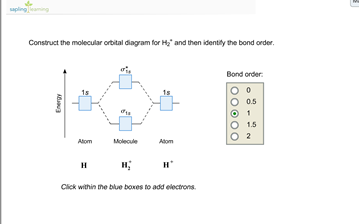
Chemistry questions and answers. Construct the molecular orbital diagram for H2+ If all of the orbitals are unoccupied, place the corresponding token in the bin underneath the answer bank. os Answer Bank 11 1 1s 1s Energy 015 all of the orbitals are unoccupied Atom Molecule Atom HT h H Identify the bond order. 0 0.5 1 1.5 2.
In this case, the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory – . Item 2: Part A Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below.M.O. diagram for N2+Molecular orbital diagram - Wikipedia

Construct The Molecular Orbital Diagram For N2 And Then Identify The Bond Order What Is The Homeworklib
To write the dot formula, construct the molecular model, and determine the molecular shape and polarity (dipole or not) for each of the following molecules or polyatomic ions. Note, with the except...
Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. 1s2, 2s2, 2p6, 3s2 ... Determine the molecular geometry of N2O (oxygen is terminal). trigonal pyramidal bent tetrahedral trigonal planar linear. ... Which molecule below …
Chapter 1 Molecular Orbital Concepts A Concepts Of Mo Theory 1 Strong Covalent Bonds Consider The Pi Bond Of Ethene In Simple Molecular Orbital Terms The Qualitative Results Would Be The Same For Any Pi Or Sigma Bond Q The Overlap Of The Two

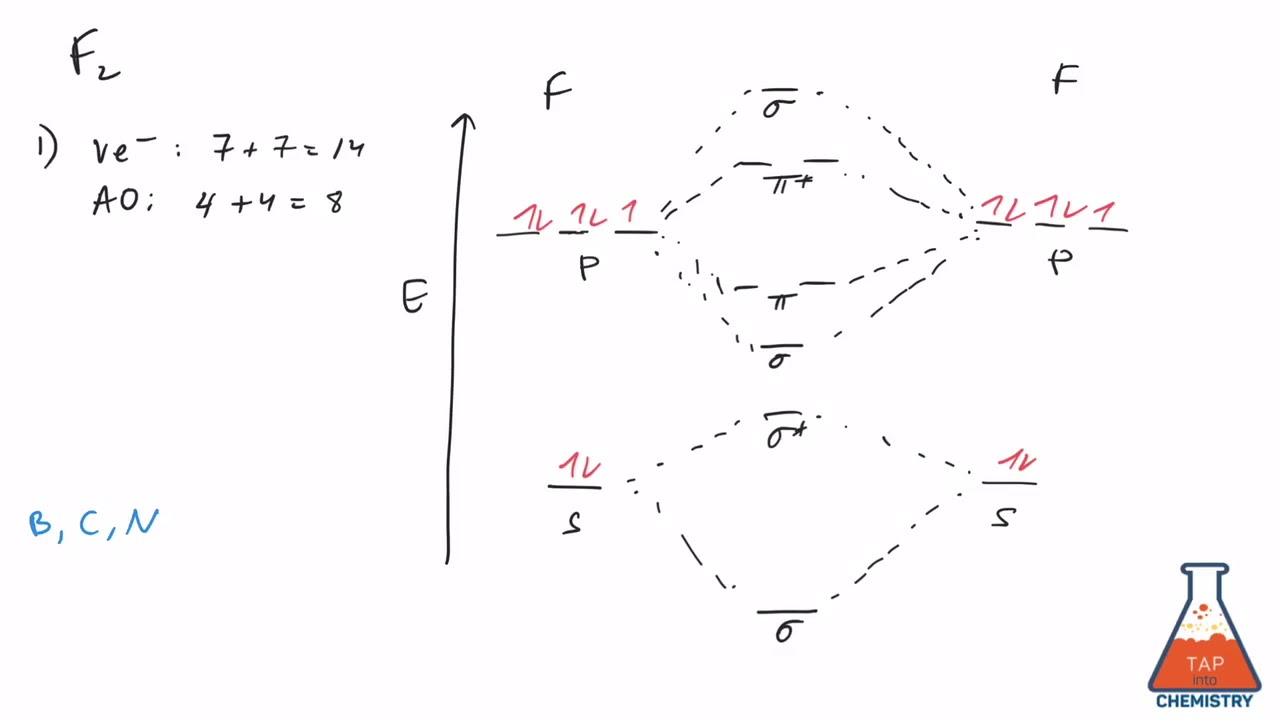
37 Draw Molecular Orbital Diagram For F2 Molecule Also Give Its Electronic Configuration Bond Order And Magnetic Property 138 Solve The Following

Draw The Molecular Orbital Diagram Of H2 02 And N2 Molecules And Calculatetheir Bond Orders As Brainly In




















0 Response to "42 construct the molecular orbital diagram for h2 below"
Post a Comment