37 Salt Dissolving In Water Diagram
Dissolving Salt in Water Dissolving Salt in Water. Just bring out a tray of interesting materials and children are excited to get busy. What about using warm water vs. cool water? Dissolving Salt in Water was super fun and engaging. You can try dissolving a variety of solids, like sugar, coffee, sprinkles, flour and more. How Does Salt Dissolve in Water? Water dissolves salt by dissociating the ions in salt from each other. Because water is a polar molecule, each of its ends holds Although common table salt easily dissolves in water, not all ionic salts do. If the strength of the attraction between the ions is much greater than the strength exerted...
Dissolving Salt in Water However, salt in cold water does not dissolve as well as if the water is warm. They will pour half of the solution in another jar and place one end of the cotton string (mop string) in each of the two jars with Epsom salt solution, as shown in the diagram on the right.

Salt dissolving in water diagram
Salinity of Water | Dissolved Salts in Sea Water Dead sea : 330000 ppm. Dissolved Salts in Sea Water. Ionic Compounds in Water - Solubility Guidelines - Guidelines or solubility rules to predict whether or not a given ionic compound is soluble in water at room temperature. Volume change on dissolving salt in water | IOPSpark When sodium chloride dissolves in water to make a saturated solution there is a 2.5 per cent reduction in volume. The solubility of salt does not change much with temperature, so there is little profit in using hot water. The salt should be in small crystals and not in rocks or very fine powder. Composition of seawater Detailed composition: abundance of the elements in seawater. Salinity: the main salt ions making the sea Dissolved gases in seawater The gases dissolved in sea water are in constant equilibrium with the In this diagram one can see how light penetrates no deeper than 150m for photosynthesis.
Salt dissolving in water diagram. Why Does Salt Dissolve In Water? How to Separate... - Koyuncu Salt Salt dissolution in water is an extremely ordinary happening that many people have not even thought about it. Yet this insignificant phenomenon is a complex process After this process, water molecules get between sodium and chlorine ions and separate them. Thus the salt is dissolved in water. Conductivity, Salinity & Total Dissolved Solids - Environmental... Salts dissolve in water to produce an anion and a cation. These ions make up the basis of conductivity in water. Ions conduct electricity due to their positive and negative charges 1. When electrolytes dissolve in water, they split into positively charged (cation) and negatively charged (anion) particles. Dissolving Salt in Water: Chemical or Physical Change? Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does... PDF Properties of solutions | Annex 1. Salt water solutions • Solids dissolved in water: the salt-water and the sugar-water systems are chosen, as the most proximate to everybody's experience. Fig. 1. Salt water solutions phase diagram at 100 kPa (NaCl - H2O). E, eutectic point. Properties of some particular solutions.
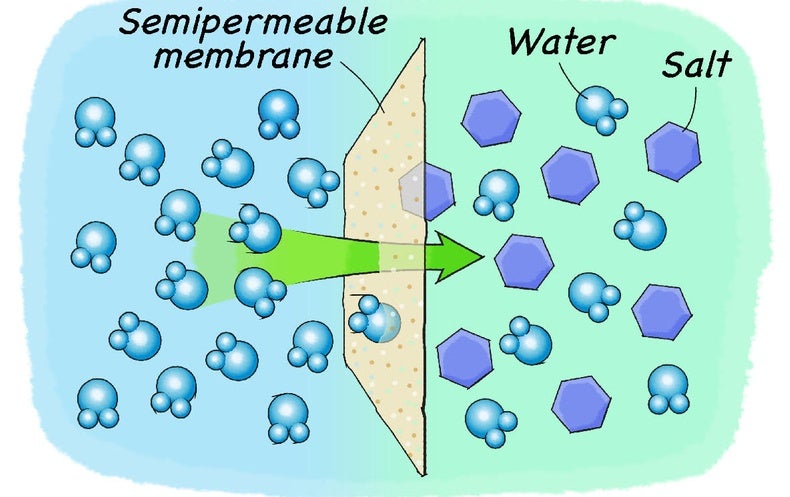
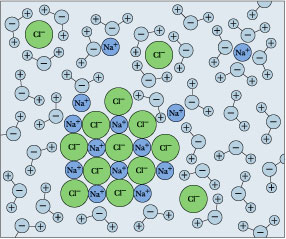
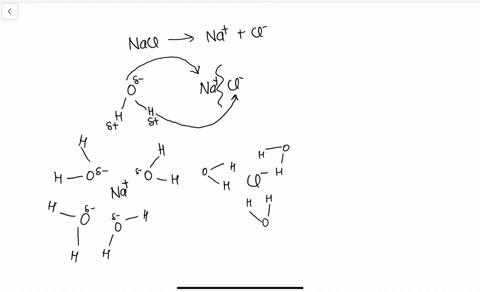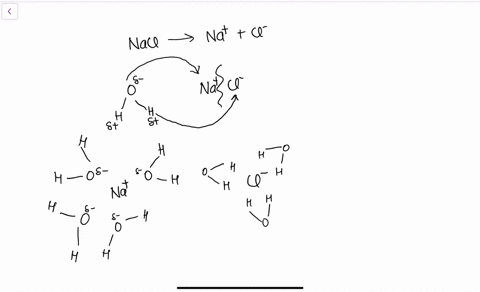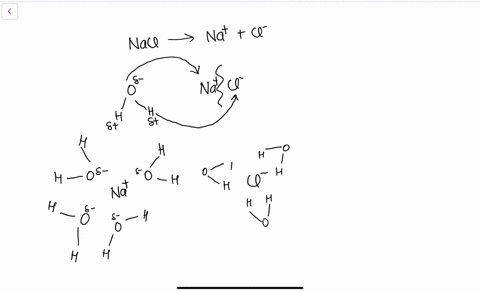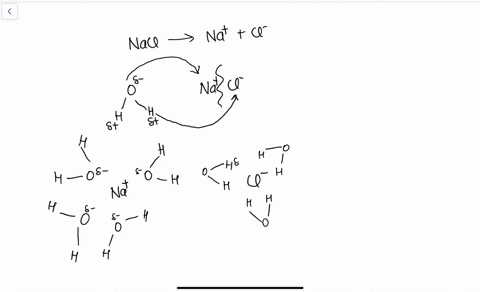
The time it takes salt to dissolve in water o f different te by Bree Farmer In conclusion the boiling water dissolved the salt the quickest which made my hypothesis correct. Aim - The aim of my experiment is to see how long it takes salt to dissolve in different temperatures of water. Hypothesis - I think that the boiling water is going to completely dissolve within 2 minutes. What Dissolves Salt Besides Water? | Sciencing Table salt is just one type of salt and is water soluble. Other water-soluble salts include nitrates, chlorides, and sulfates. There are exceptions to the rule, though. A salt is considered insoluble if, by Purdue University's definition, it can dissolve in room temperature water to a concentration of... The diagram shows salt dissolved in water. What does... - Brainly.com What does it show about water molecules and chloride ions? + Chloride Oxygen Hydrogen 4 Sodium. that they all dissolved went on watef. Image of Molecules from Salt Dissolving in Water Animation Animations and simulations are dynamic visualizations as opposed to static visualizations (e.g. diagrams, etc.). The goal of this chapter is to show how students use dynamic visualizations to internalize concepts and imagery and to explore chemical phenomena.
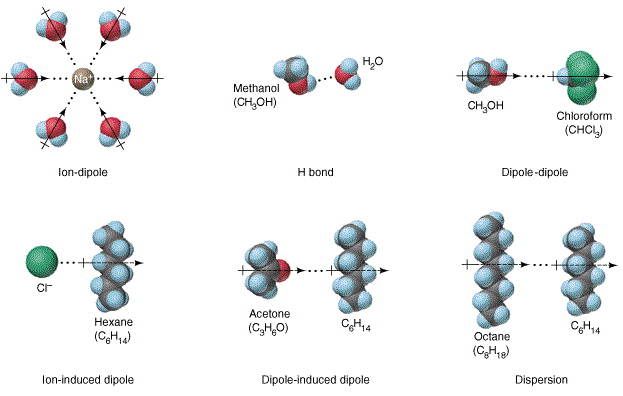
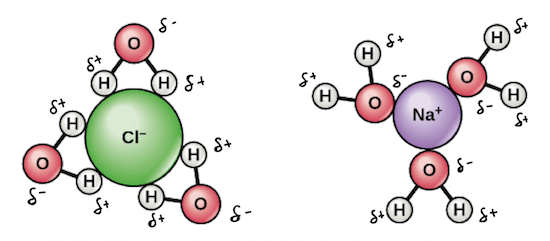
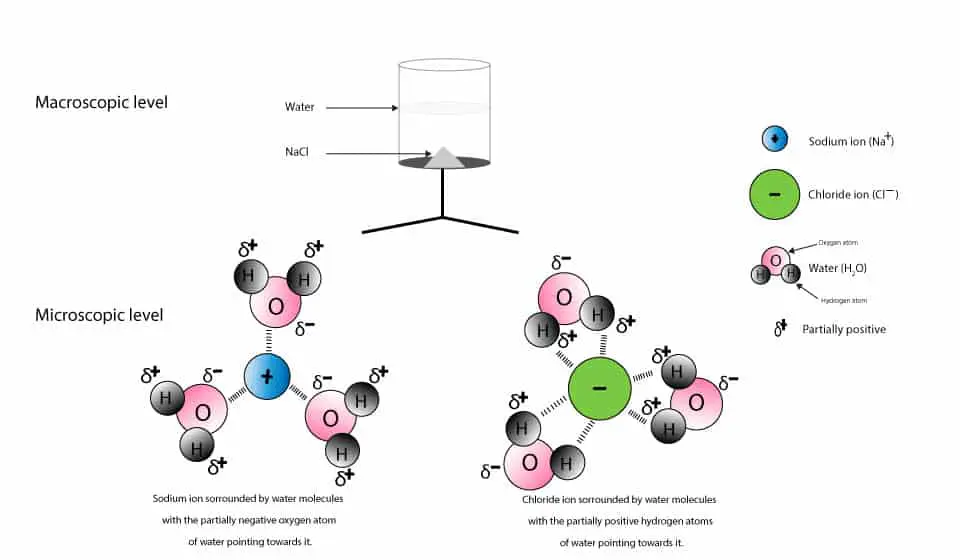
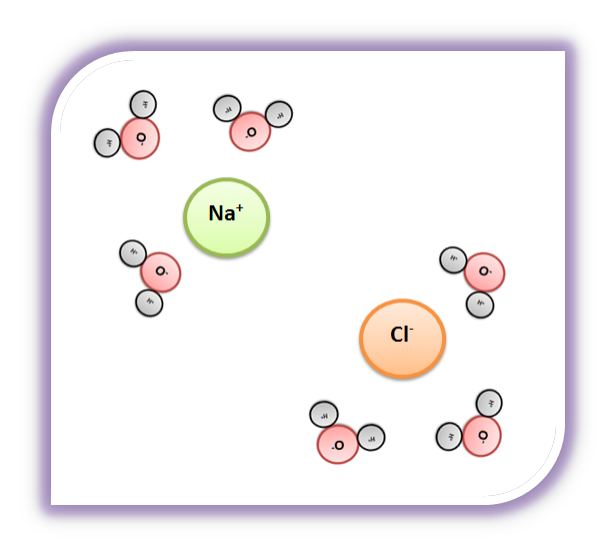
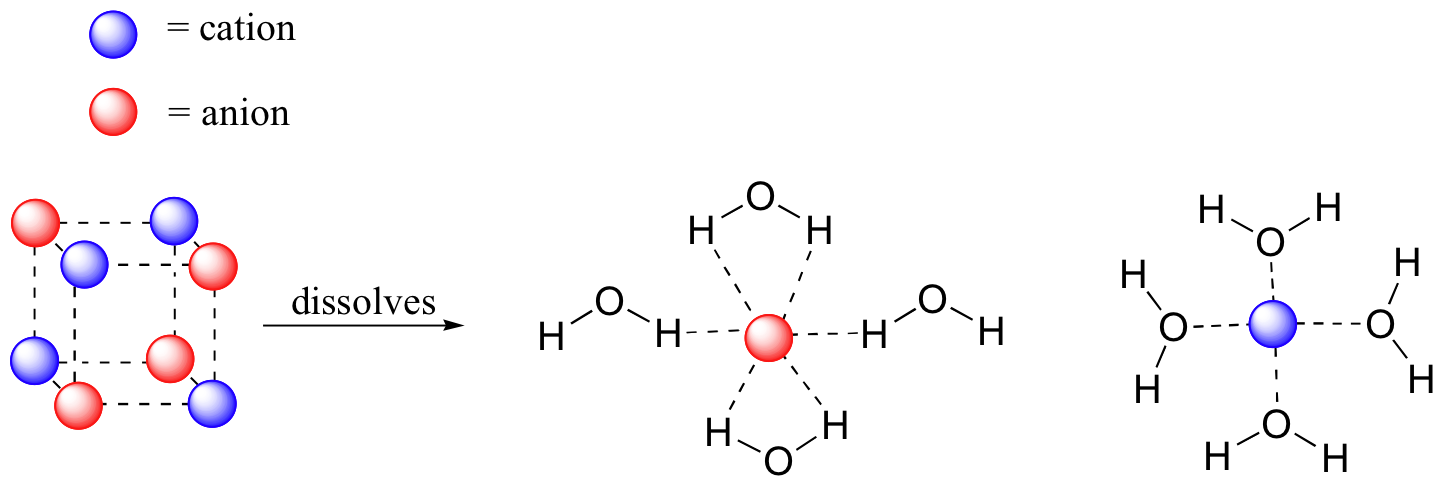
Why Does Salt Dissolve in Water But Not Oil? | LEAFtv Why Salt Dissolves in Water. Salt or sodium chloride consists of sodium and chloride ions joined by an ionic bond to form a charged NaCl molecule. Why Salt Does Not Dissolve in Oil. Oil molecules do not contain any charge. Oil is comprised of long chains of hydrogen and carbon atoms linked to each... If salt has been dissolved in water, is it in a solid or liquid state? When a salt is dissolve in water, it is completely ionized in water and produces cations and anions. Water can dissolve salt because the positive part of water molecules attracts the negative chloride To fully understand the phenomenon, look up the sodium chloride (salt)/water phase diagram and its... How to Dissolve Salt in Water: 9 Steps (with Pictures) - wikiHow Pour the salt into the water. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. You need water molecules to come in contact with your salt to dissolve it, and stirring The amount of salt you can dissolve in water depends on a few variables like temperature and purity. A level What happens when an ionic compound dissolves in water... When salts dissolve in water a process of solvation occurs in which the ions become solvated by association with the solvent molecules. In the case of anions, the positive ends of the water molecules (ñHδ+) will orientate themselves towards the negative anion and the water molecules...
When common salt is dissolved in water | Toppr Ask A solution of this salt was prepared by dissolving 0.25g of this sodium salt of protein in 10g of water and ebulliscopic analysis revealed that solution boils at temperature 5.93×10−3∘C higher than the normal boiling point of pure water. Kb of water 0.52kgmol−1.
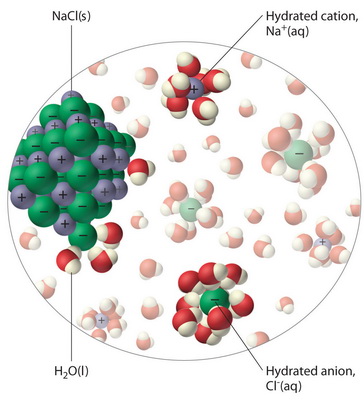
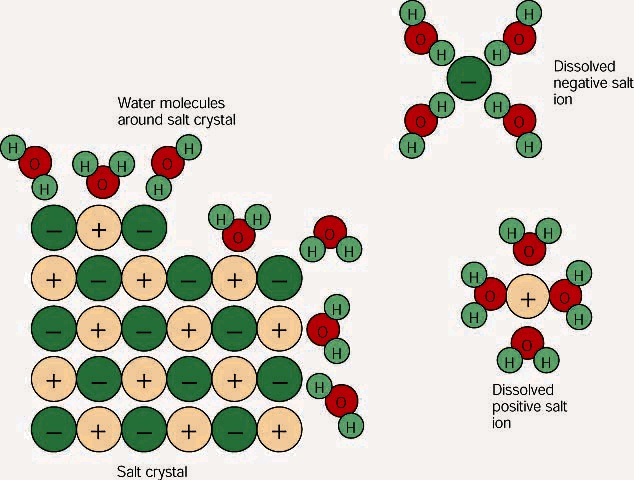
Why does salt crystals dissolve in the water? | Wyzant Ask An Expert Salt dissolution in water is an extremely ordinary happening that many people have not even thought about it. Yet this insignificant phenomenon is a complex process After this process, water molecules get between sodium and chlorine ions and separate them. Thus the salt is dissolved in water.
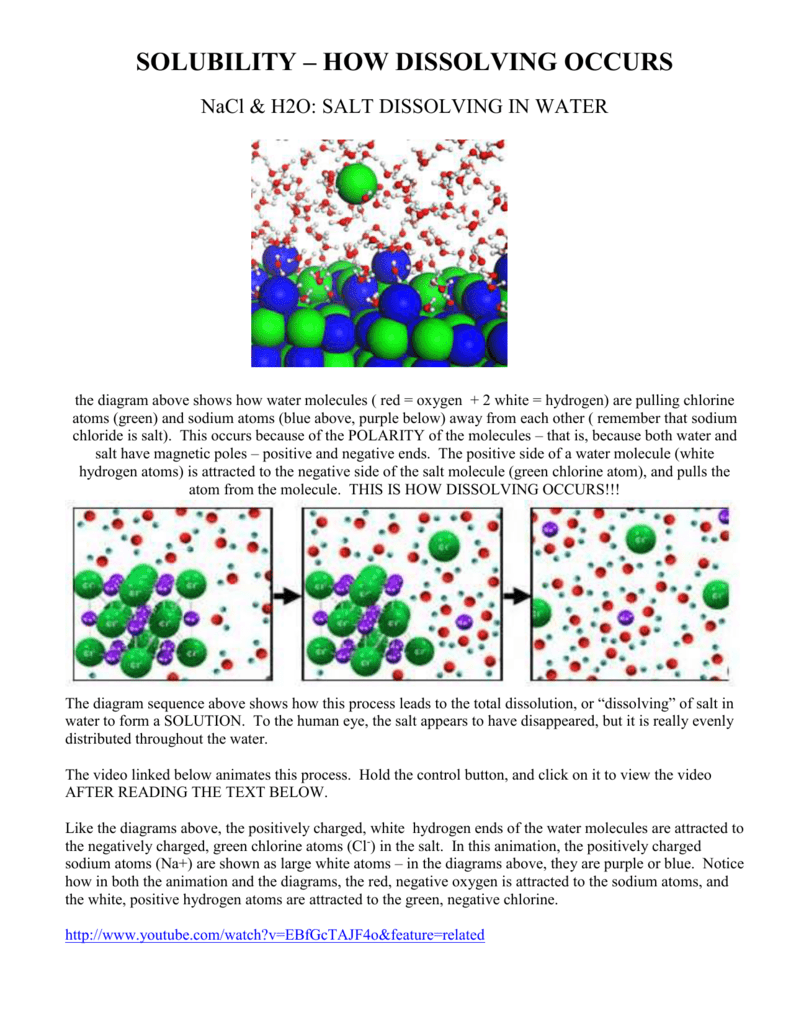
solubility - how dissolving occurs SOLUBILITY - HOW DISSOLVING OCCURS NaCl & H2O: SALT DISSOLVING IN WATER the diagram above shows how water molecules ( red = oxygen + 2 white = hydrogen) are pulling chlorine atoms (green) and sodium atoms (blue above, purple below) away from each other...
Dissolving and Back Again - American Chemical Society Students dissolve salt in water and allow the water to evaporate to investigate the question: What process causes salt to Students will be able to develop and explain a particle-level model to describe their observations of water dissolving salt, the water evaporating, and the salt crystals re-forming.
Which Solids Dissolve In Water - Cool Science for Kids Things like salt, sugar and coffee dissolve in water. They are soluble. They usually dissolve faster and better in warm or hot water. Solids dissolve faster in hot water as in hot water the water molecules are moving faster, so bump into the solid more often which increases the rate of reaction.
Saline water - Wikipedia Saline water (or salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride). The salt concentration is usually expressed in parts per thousand (permille, ‰) and parts per million (ppm).
Dissolved Salt - an overview | ScienceDirect Topics The presence of dissolved salts in the water will accelerate the corrosion process by the provision of ions available to effect a more rapid neutralisation of Polystyrene divinyl benezene and carboxylic-based resins are the primary materials used in water treatment. The resins are further classified as...
Why Does Water Dissolve Salt? | Chapter... | Middle School Chemistry Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions and the negative part of water molecules attracts the Project an image and have students model what happens when salt dissolves in water. Show students a series of four pictures to help explain...
Water molecules and their interaction with salt | U.S. Geological Survey At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.
solid-liquid phase diagrams: salt solution Shows how the phase diagram for mixtures of salt and water is built up, and how this leads to a eutectic mixture of salt and water. A solution is saturated if it won't dissolve any more of the salt at that particular temperature - in the presence of crystals of the salt.
Salinity - Wikipedia Salinity (/səˈlɪnɪti/) is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). This is usually measured in. (note that this is technically dimensionless).
Composition of seawater Detailed composition: abundance of the elements in seawater. Salinity: the main salt ions making the sea Dissolved gases in seawater The gases dissolved in sea water are in constant equilibrium with the In this diagram one can see how light penetrates no deeper than 150m for photosynthesis.
Volume change on dissolving salt in water | IOPSpark When sodium chloride dissolves in water to make a saturated solution there is a 2.5 per cent reduction in volume. The solubility of salt does not change much with temperature, so there is little profit in using hot water. The salt should be in small crystals and not in rocks or very fine powder.
Salinity of Water | Dissolved Salts in Sea Water Dead sea : 330000 ppm. Dissolved Salts in Sea Water. Ionic Compounds in Water - Solubility Guidelines - Guidelines or solubility rules to predict whether or not a given ionic compound is soluble in water at room temperature.


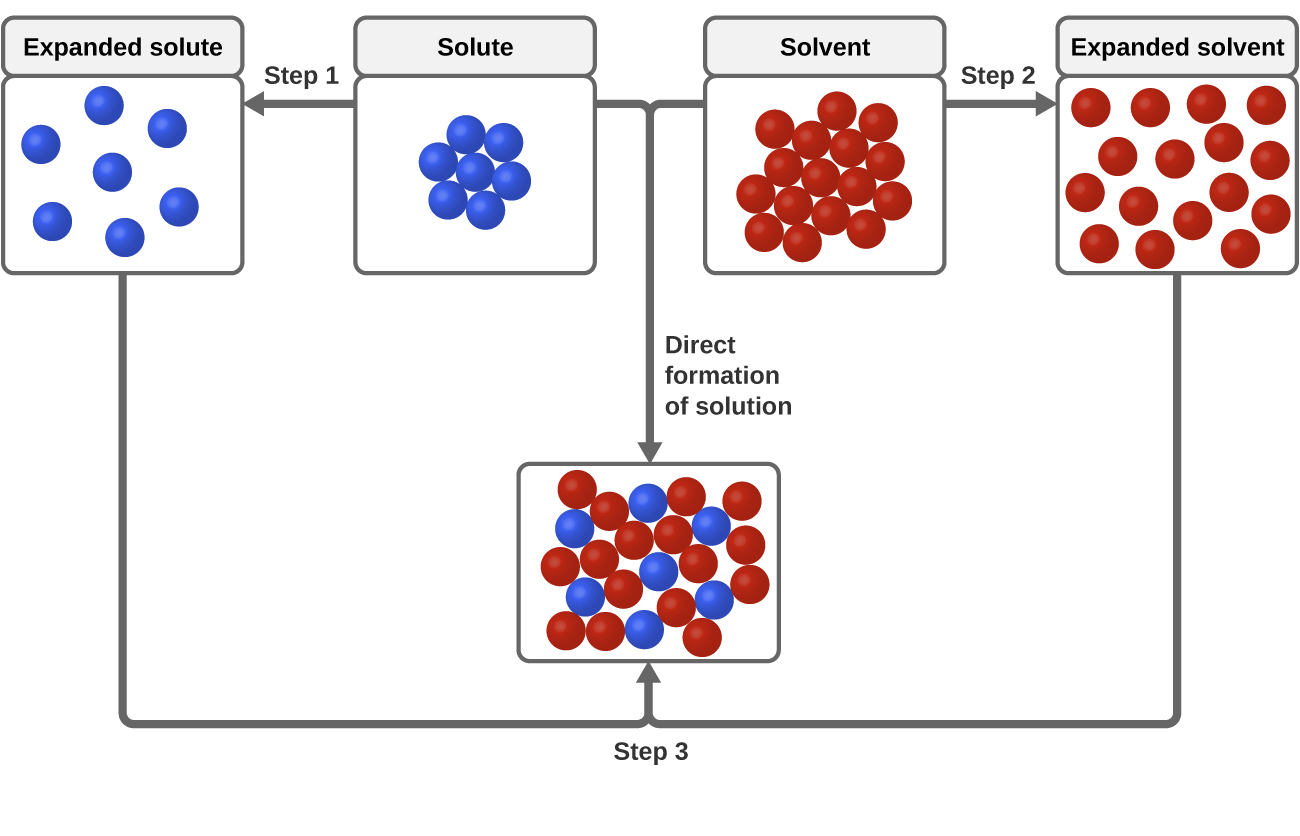
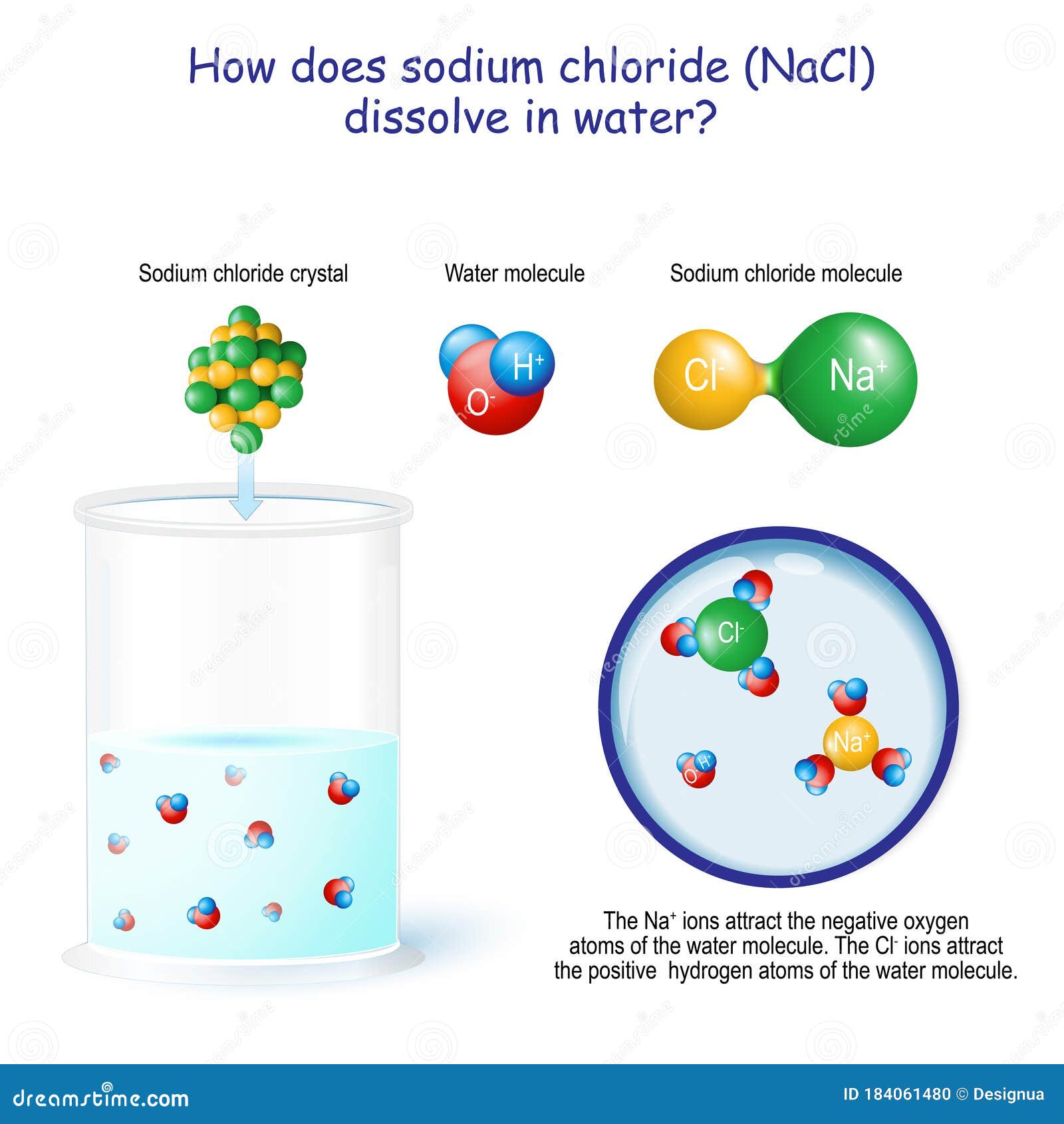











0 Response to "37 Salt Dissolving In Water Diagram"
Post a Comment