41 boron lewis dot diagram
Boron Lewis Dot Structure | Borates Today Lewis dot structure is the structure of an element or molecule, and total valence electrons are as dots to represent the bond pairs and lone pairs. Boron electronic configuration counts as 2,3, its atomic number 5. Hence, it has three electrons in the valence shell. BCl3 Lewis Structure, Molecular Geometry, and ... BCl3 Lewis Structure. Let us apply the lewis dot rules and try to draw the structure of boron trichloride. First of all, we need to calculate the total valence electrons of this molecule, B = 3. C l= 7. 3Cl = 7*3=21. So, total= 21+3= 24. Now, boron is less electronegative, which makes it the central atom.
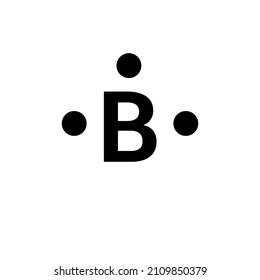
Lewis dot structure of boron? - Answers the Lewis structure of B or Boron would have three small dots posing as electrons. These dots can be placed anywhere around the B symbol.

Boron lewis dot diagram
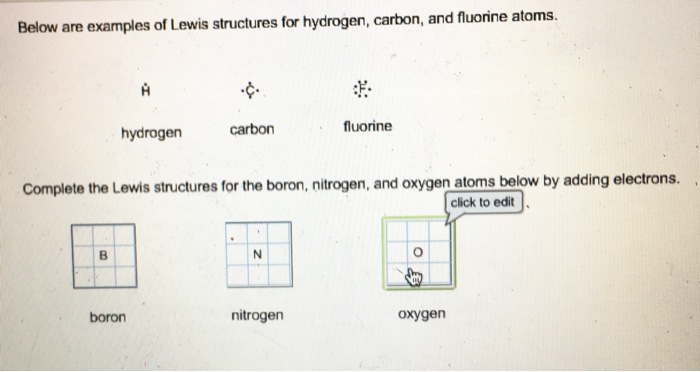
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Boron Electron Dot Diagram - xxjasela #Boron Electron Dot Diagram How To Draw Lewis; Its atomic mass is 10.81 grams per mole. The physical properties of the molecule (like boiling point, surface tension, etc.).Boron is an element in the periodic table with a symbol B 5 is its atomic number. cosmo-kasino350.de › csbr-lewis-structureCO Csbr lewis structure. CO . CH3OH I2 . B r −
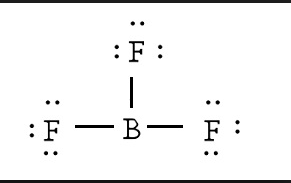
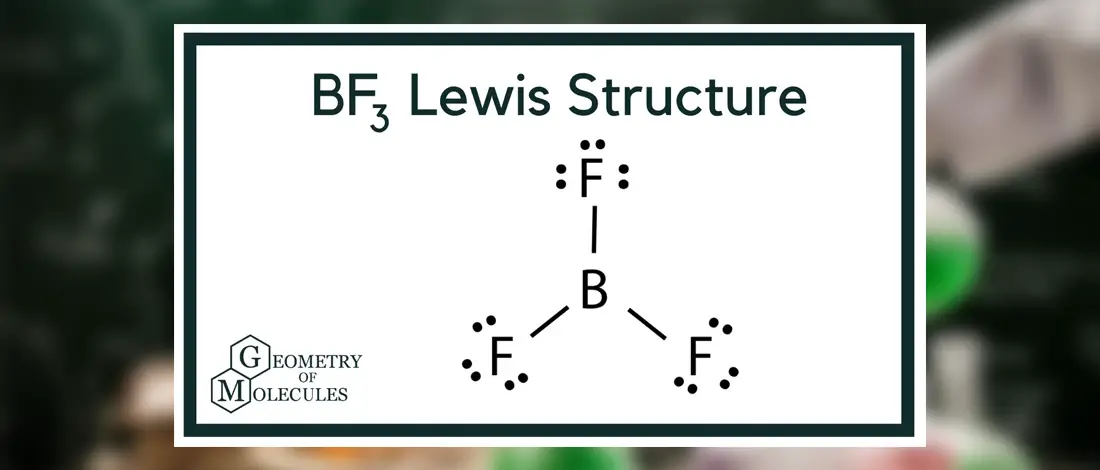
Boron lewis dot diagram. Boron triiodide (BI3) lewis dot structure, molecular ... BI3 lewis structure contains three B-I bonds with boron in a central position and all three iodine atoms in an outside position. The lewis structure of BI3 contains a total of 3 bond pairs and 9 lone pairs. The drawing of the BI3 lewis's structure is very easy and simple. Let's see how to do it. techiescientist.com › clf5-lewis-structureClF5 Lewis Structure, Molecular Geometry ... - Techiescientist 2 days ago · Lewis Structure is also known as an electron-dot structure since it uses dot notations to represent the valence shell electrons in the skeletal diagram. Here, as we can see, we have put all the 42 electrons surrounding the six atoms in ClF5. Since Chlorine is the central atom here, it will form bonds with all the five Fluorine atoms. Lewis Structure of Boron Trifluoride (BF3) BF 3 lewis structure. According to the lewis structure of BF 3, there are only six electrons around boron atom.Therefore, octal of boron atom is not completed. Therefore, borane BF 3 is considered as a lewis acid.. Steps of drawing lewis structure of BF 3. There are general guidelines to draw a lewis structure step by step and they are mentioned below. Lewis Structure of Borane (BH3) - chemistryscl.com Lewis Structure of Borane (BH. 3. ) Borane (BH 3) is a lewis acid and there are one boron atom and three hydrogen atoms in borane molecule. Each hydrogen atom has connected with boron through a single bond in the lewis structure of borane (BH3). There are only three bonds around boron atom and no lone pairs on boron atom.
Lewis Dot Structure for Boron Atom (B) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for B (Boron). I show you where Boron is on the periodic table and how to determine how ma... Why is Boron Trifluoride written in two ways in the lewis ... Its formula is = [no. of valence electrons on atom] - [non-bonded electrons + number of bonds]. or a simple one {if no lone pair are there} = 1/2 no. of bonds.)on each atom. Note:Boron disobeys octet rule in Lewis structure. Its a property of it about which we cannot do anything. (in fig. 1). We must examine the formal charges of this structure. Lewis Electron- Dot Structure of boron fluoride BF ... Lewis Electron- Dot Structure of boron fluoride BF molecule - #41 A simple and general method for writing Dot Structures - Lewis Structures is given in a previous article entitled "Lewis Structures and the Octet Rule". Relevant worked examples were given in the following articles: ... Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
› watchVSEPR Theory: Introduction - YouTube To see all my Chemistry videos, check outhttp://socratic.org/chemistryThis is an introduction to the basics of VSEPR Theory. VSEPR theory is a set of rules f... Lewis Dot Structure for Boron Oxide B2O3 Boron metal has 3 ... Lewis Dot Structure for Boron Oxide (B2O3) Boron (metal) has 3 valence electrons Oxygen (nonmetal) has 6 valence electrons, needs 2 to be balanced B3↘ ↙O2 B2 O3 Lewis Dot Structure for Sodium Fluoride Na +1 Cl-1 Na has 1 valence electron Fl needs 1 to be balanced Na Cl Covalent Bonds Covalent Bonds are attractions of 2 nonmetals They have low melting/boiling points and aren't a good ... BF3 Lewis Structure (2022 UPDATED) Practical Guide In BF3, Boron is the least electronegative atom and will be used as the central atom. The Lewis Dot Structure will show you one Boron atom with three electrons in its last shell and three Fluorine atoms with seven electrons in its last shell. The computation will end with 24 total valence electrons, forming three B F bonds. en.wikipedia.org › wiki › Lewis_acids_and_basesLewis acids and bases - Wikipedia Some of the most studied examples of such Lewis acids are the boron trihalides and organoboranes, but other compounds exhibit this behavior: BF 3 + F − → BF 4 −. In this adduct, all four fluoride centres (or more accurately, ligands) are equivalent. BF 3 + OMe 2 → BF 3 OMe 2. Both BF 4 − and BF 3 OMe 2 are Lewis base adducts of boron ...
BCl3 Lewis Structure ||Lewis Dot Structure for BCl3 ... BCl3 Lewis Structure ||Lewis Dot Structure for BCl3 ||Boron Trichloride Lewis Structure||Lewis structure for BCl3 with formal charges#BCl3LewisStructure#Lewi...
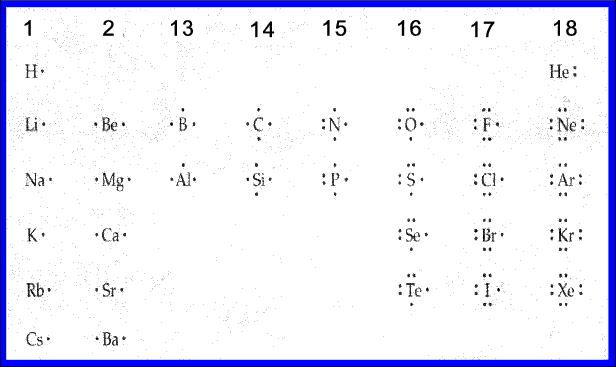
opentextbc.ca › lewis-electron-dot-diagramsLewis Electron Dot Diagrams – Introductory Chemistry – 1st ... A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Boron Lewis Dot Structure: Drawing, Several Compounds and ... Boron Lewis dot structure for Boron trifluoride. Fluorine is also a halogen element like chlorine. Therefore, The properties shown by Boron trifluoride is quite similar as boron trichloride. The Lewis Dot structure would identify the similarities with proper description. Fluorine is the least electronegative element in the periodic able. Therefore.
Boron tribromide (BBr3) lewis dot structure, molecular ... Boron tribromide (BBr3) lewis dot structure, molecular geometry, polar or nonpolar, hybridization Home > Chemistry Article > BBr3 lewis structure and its molecular geometry Boron tribromide composed of boron and bromine appears as colorless to amber liquid, has a sharp and irritating odor with chemical formula BBr3.
Ch4 Lewis Dot Structure - solved name formula ch4 h20 ... Ch4 Lewis Dot Structure - 18 images - ch3 lewis structure, lewis dot structures neutral compounds chemistry video, lewis diagram for hcn, 29 lewis dot structure of ch4 how to draw lewis,
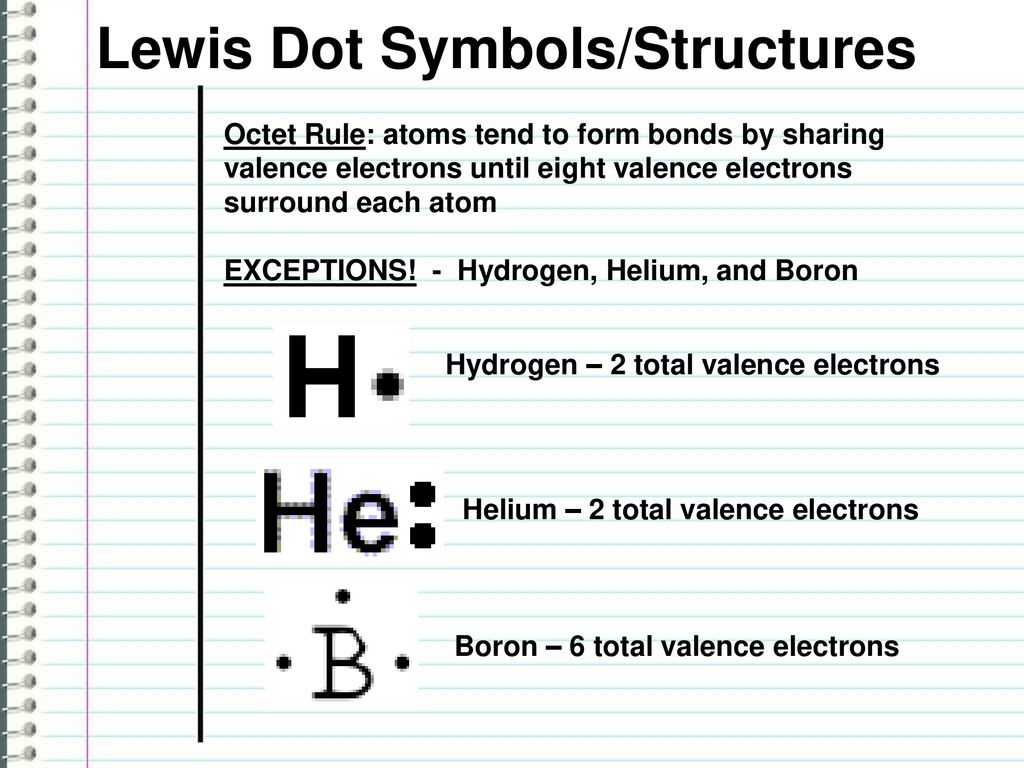
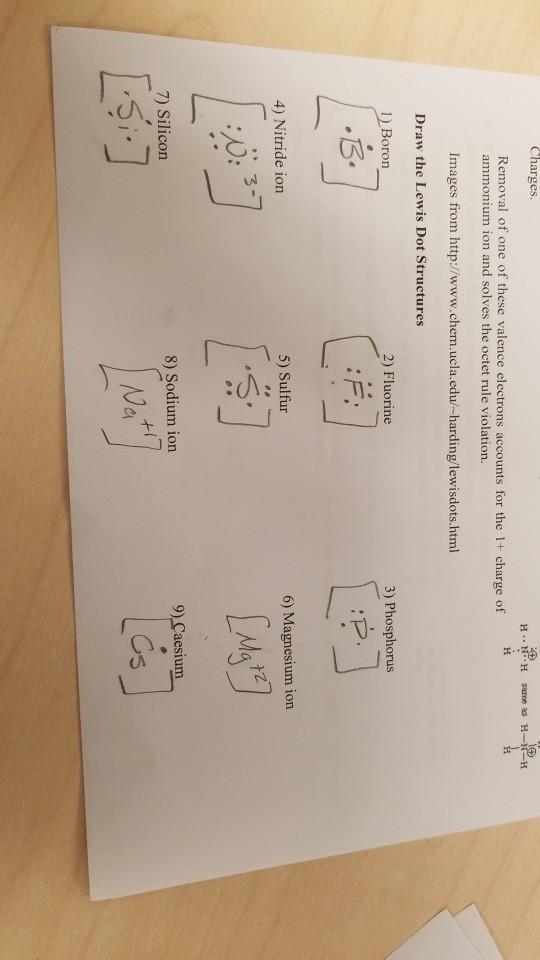
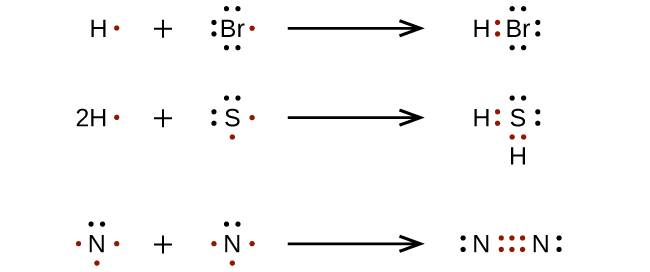
The three dots in the Lewis dot diagram for boron ... The three dots in the Lewis dot diagram for boron indicates that it has three valence electrons.. The three dots in the Lewis dot diagram for boron indicates that it. can bond with three other atoms.NO.It can bond with less than 3 atoms by forming double or triple bonds.; can only form triple covalent bonds.NO.It can also form single and double covalent bonds.; has three valence electrons.
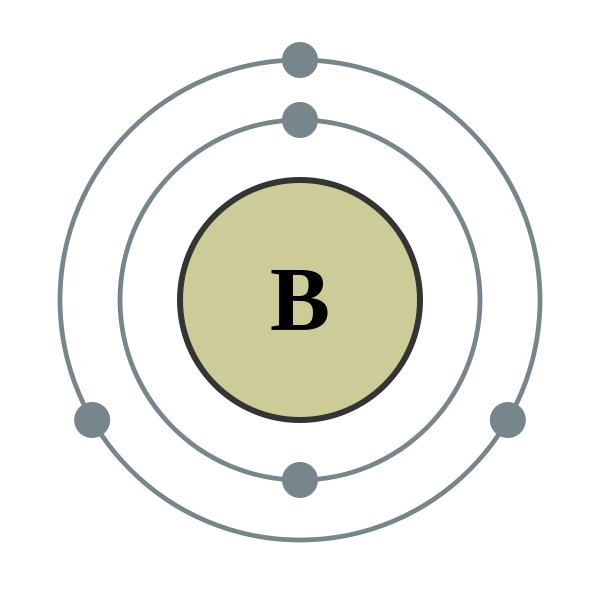
Lewis Dot Diagram For Boron An electron 13, Electron dot diagram for boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;. Structure, properties, spectra, suppliers and links for: Boron nitride. Boron has 5 electrons. 3 are in the valence shell.
Lewis Dot Diagram - Organic Chemistry - Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
How to Draw the Lewis Dot Structure for BN: Boron nitride ... A step-by-step explanation of how to draw the BN Lewis Dot Structure (Boron nitride).For the BN structure use the periodic table to find the total number of ...
Lewis Dot Structure of BCl3 (Boron TriChloride) - YouTube I quickly take you through how to draw the Lewis Structure of BCl3, (Boron TriChloride). I also go over formal charge, hybridization, shape and bond angle.
How to draw BBr3 Lewis Structure? - Science Education and ... To sketch the BBr3 Lewis structure by following these instructions: Step-1: BBr3 Lewis dot Structure by counting valence electrons on the boron atom. Step-2: Lewis Structure of BBr3 for counting valence electrons around the terminal bromine atom. Step-3: Lewis dot Structure for BBr3 generated from step-1 and step-2.
topblogtenz.com › oxygen-bohr-modelOxygen Bohr Model - How to draw Bohr diagram for Oxygen(O) atom Electron dot diagram of the Oxygen atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Oxygen, we got to know, it has 6 valence electrons. So, just represent these 6 valence electrons around the Oxygen atom as a dot.
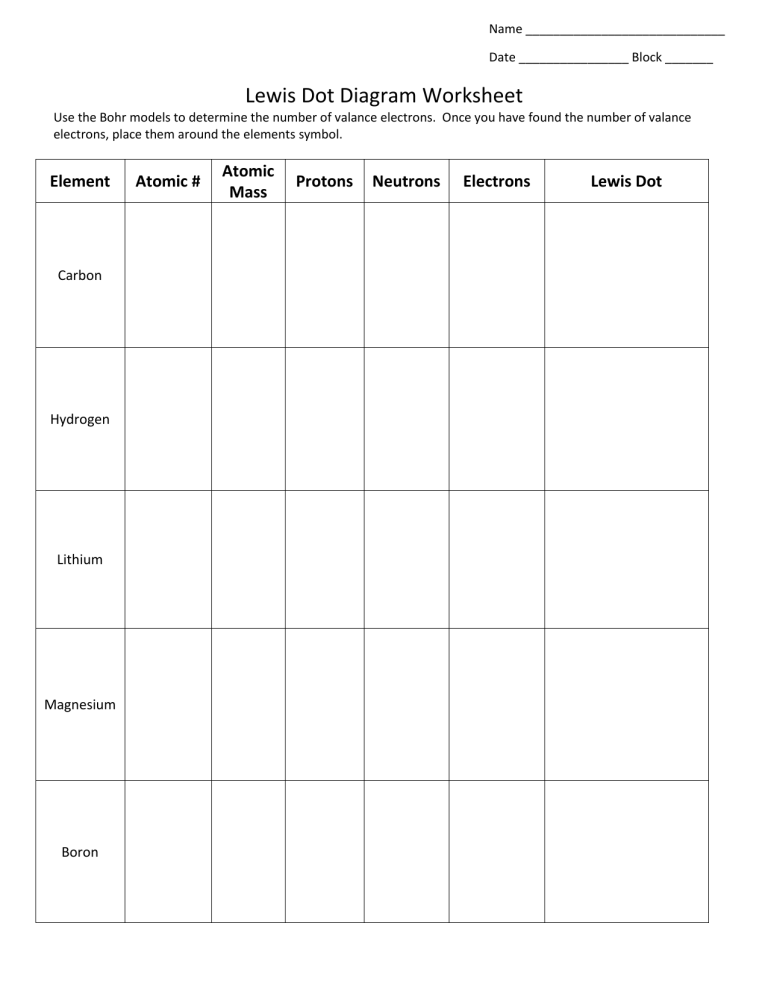
› cms › libAtomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon Hydrogen Lithium Magnesium Boron
Lewis Dot Structure for Boron||How do you draw the Lewis ... Lewis Dot Structure for Boron||How do you draw the Lewis Dot structure for Boron atom?||Lewis Symbol for Boron
BF3 Lewis Structure, Molecular Geometry, and Hybridization BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ...
cosmo-kasino350.de › csbr-lewis-structureCO Csbr lewis structure. CO . CH3OH I2 . B r −
Boron Electron Dot Diagram - xxjasela #Boron Electron Dot Diagram How To Draw Lewis; Its atomic mass is 10.81 grams per mole. The physical properties of the molecule (like boiling point, surface tension, etc.).Boron is an element in the periodic table with a symbol B 5 is its atomic number.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...









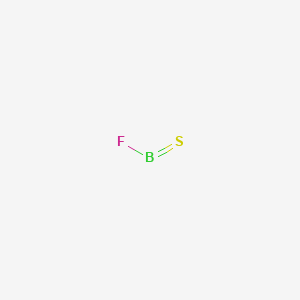



/Lewis-dot-58f78f405f9b581d5938e617.jpg)
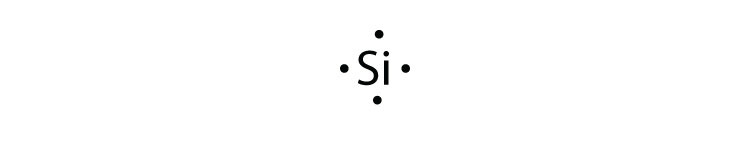



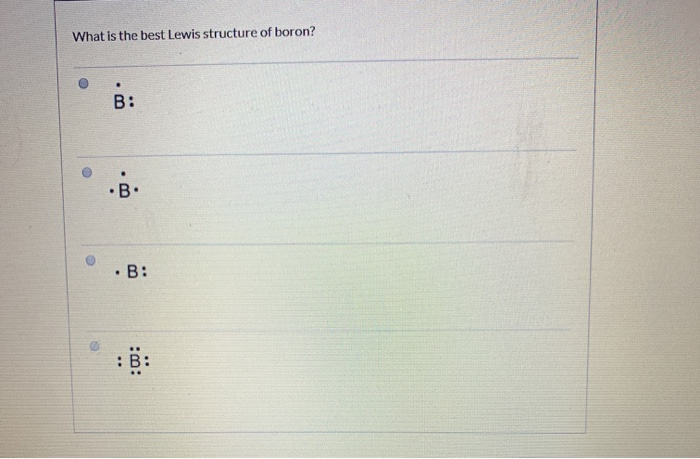
![Best Answer] Choose the write Lewis electron dot diagram for ...](https://us-static.z-dn.net/files/d88/47b81ffdeb97e849146b837bc52b2301.png)


0 Response to "41 boron lewis dot diagram"
Post a Comment