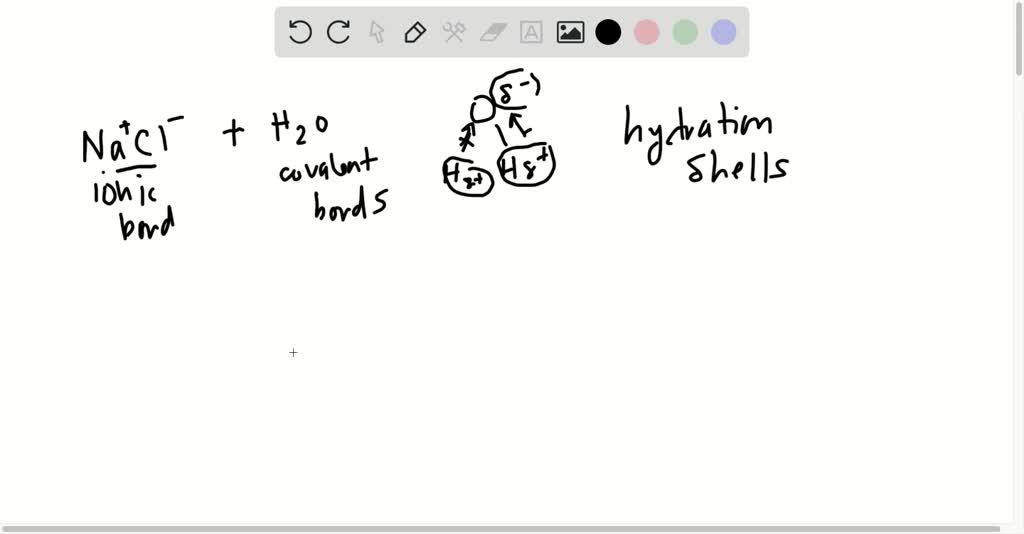
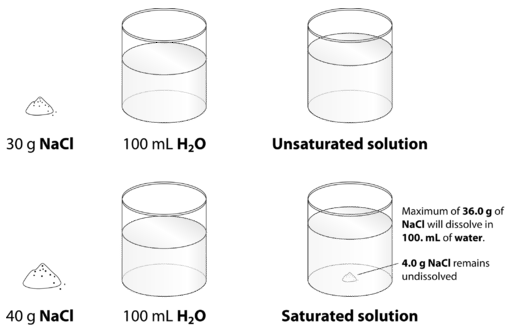
38 nacl dissolved in water diagram
How does water dissolve salts These free ions in a salt-water solution allow electricity to flow through water. Ionic compounds such as sodium chloride, that dissolve in water and dissociate to form ions, are called electrolytes. Please Watch animation 10.3 on ionic solutions. Hypotonic Solution Examples & Diagram | What is a ... 22.5.2021 · A hypotonic solution example is salt water. The salt is the solute, ... The diagram below shows a cell in a hypotonic environment. ... Solutions are made of solute dissolved in a …
The Enthalpy Change of Sodium Chloride Added to Water ... The Enthalpy Change of Sodium Chloride Added to Water. When salt dissolves in water, sodium and chloride ions are pulled apart to form new weak bonds with water molecules. Pulling them apart takes energy, while forming new bonds with the water molecules releases energy. The net amount of energy released or absorbed is ...

Nacl dissolved in water diagram
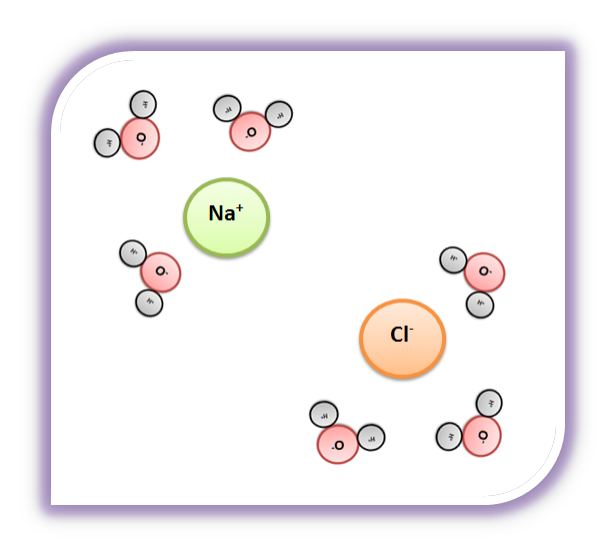
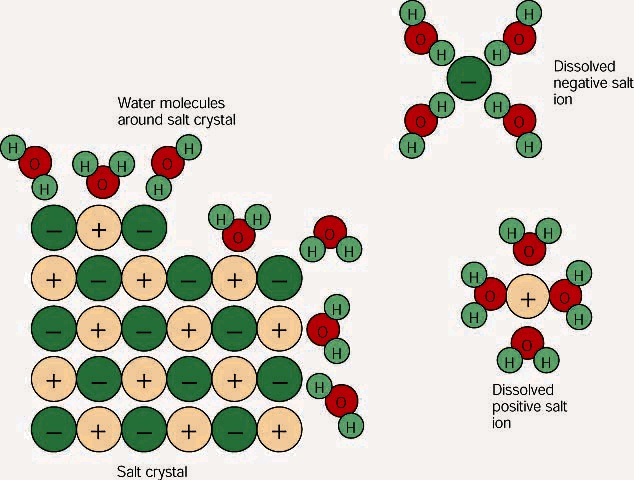
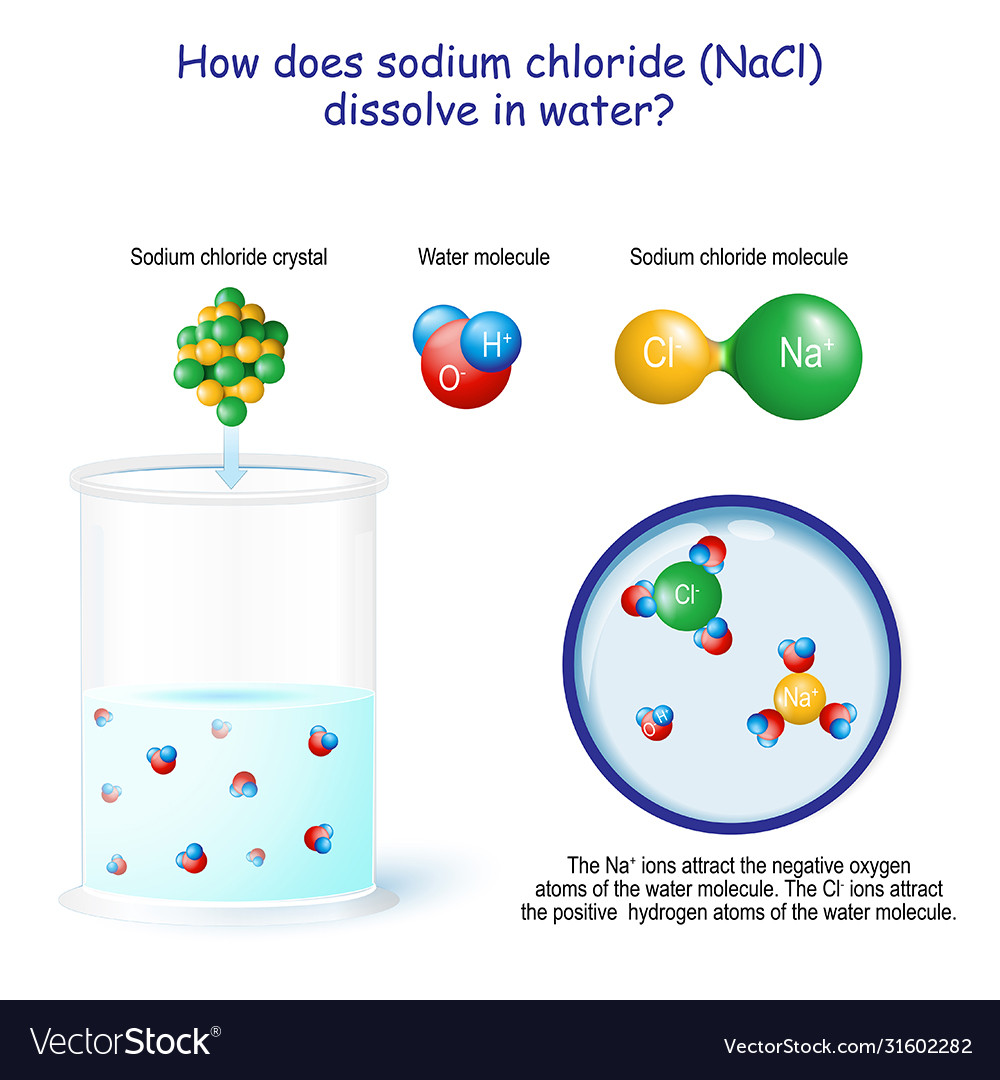
SOLVED:Draw a diagram of table salt (NaCl) dissolved in water. Video Transcript. we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. And chloride has one extra electrons in the outer plowed compared ... What happens when sodium chloride dissolves in water ... What happens when sodium chloride dissolves in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in ... What happens when sodium chloride NaCl is dissolved in water? What happens when sodium chloride NaCl is dissolved in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, […]
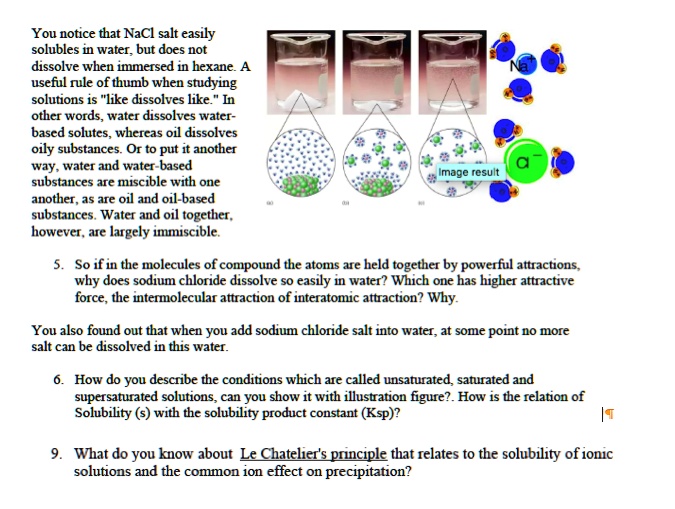
Nacl dissolved in water diagram. 44 diagram of salt dissolved in water - Wiring Diagram Source Jan 23, 2022 · These free ions in a salt-water solution allow electricity to flow through water. Ionic compounds such as sodium chloride, that dissolve in water and dissociate to form ions, are called electrolytes. Please Watch animation 10.3 on ionic solutions. Diagram of salt dissolved in water. Salt Dissolved In Water: Molecular Interactions. How does sodium chloride (NaCl) dissolve in water ... Oct 24, 2017 · Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl –) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed. Water (data page) - Wikipedia Up to a temperature of 0.01 °C, the triple point of water, water normally exists as ice, except for supercooled water, for which one data point is tabulated here. At the triple point, ice can exist together with both liquid water and vapor. At higher temperatures, the … pH of NaCl — Acidic, Basic or Neutral - Techiescientist Therefore, by the calculations, it is proved that the pH of sodium chloride when dissolved in water at room temperature i.e at 25°C, is equal to 7. Structure of NaCl As NaCl is an inorganic compound, its structure cannot be explained by the rules used to understand the structures of organic compounds.
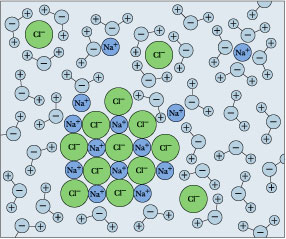
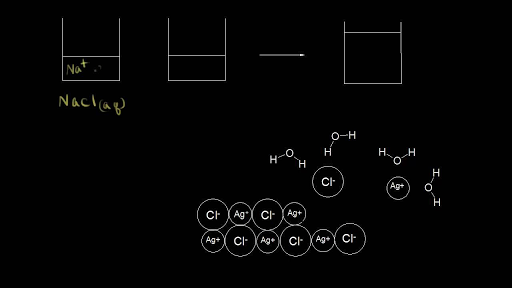
TYPES OF SOLUTIONS tested using a conductivity apparatus (diagram below). Electrolytes are further classified as strong electrolytes and weak electrolytes. In water, a strong electrolyte exists only as ions. Strong electrolytes make the light bulb on the conductivity apparatus glow brightly. Ionic substances such as NaCl are strong electrolytes. SOLVED:List the three steps that occur as \mathrm{NaCl ... we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. And chloride has one extra electrons in the outer plowed compared to the neutrons, protons that are in the nucleus with you and ... Solved: Which of the following diagrams best represents ... 100% (7 ratings) for this solution. Step 1 of 5. If a strong electrolyte dissolves in water, it completely dissociates into ions. If a weak electrolyte dissolves in water, it's dissociation is less. NaCl is a strong electrolyte. Hence, it completely dissociates in to ions in water. Chapter 4, Problem 8P is solved. NaCl is dissolved in water. Which of the following ... Sodium chloride (N a C l) is a strong electrolyte. When dissolved in water, it dissociates into sodium ion (N a +) and chloride ion (C l −), water surrounds each ion. Thus, sodium ion (N a +) and chloride ion (C l −) is hydrated.
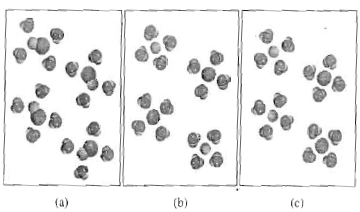
Solid-liquid Phase Diagrams: Salt Solution At all temperatures above that marked on the graph (about 57°C), 100 g of water will dissolve more than 100 g of potassium nitrate. All the potassium nitrate will stay in solution. At 57°C, you hit the solubility curve. This is the temperature at which 100 g of water will dissolve 100 g of potassium nitrate to give a saturated solution. What Is The Reaction Of NaCl With Water What is the reaction of NaCl with water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous ... Why does methanol dissolve well in water. Explain using a ... A. dissolve 5.8 moles of NaCl in 1 L of water B. dissolve 1.85 moles of NaCl in . chemistry. the autoionization of water, as represented by the equation below, is known to be endothermic. What can be correctly said of what occurs as the temperature of water is raised?(please explain) H2O(l)+H2O(l)H3O+(aq) + OH-(aq) What is the dissociation equation for NaCl? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Why Does Water Dissolve Salt? | Chapter 5: The Water ... Project an image and have students model what happens when salt dissolves in water. Show students a series of four pictures to help explain the process of water dissolving salt. Project the image Sodium Chloride Dissolving in Water. Point out that several water molecules can arrange themselves near an ion and help remove it from the crystal.
what happens when nacl is dissolved in water - Lisbdnet.com 6 Dec 2021 — After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this ...
What happens when sodium chloride is dissolved in water ... Aug 07, 2020 · What happens when sodium chloride is dissolved in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting ...
Water molecules and their interaction with salt - USGS Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Solubility - Wikipedia However, there is a limit to how much salt can be dissolved in a given volume of water. This concentration is the solubility and related to the solubility product, K sp. This equilibrium constant depends on the type of salt (AgCl vs. NaCl, for …
PDF Ap Chemistry Review Particulate Diagrams 2. The diagram above represents a mixture of NO 2(g) and N 2O 4(g) in a 1.0 L container at a given temperature. The two gases are in equilibrium according to the equation 2 NO 2(g) N 2O 4(g) Which of the following must be true about the value of the equilibrium constant for the reaction at this temperature? (A) K = 0 (B) 0 < K < 1 (C) K = 1
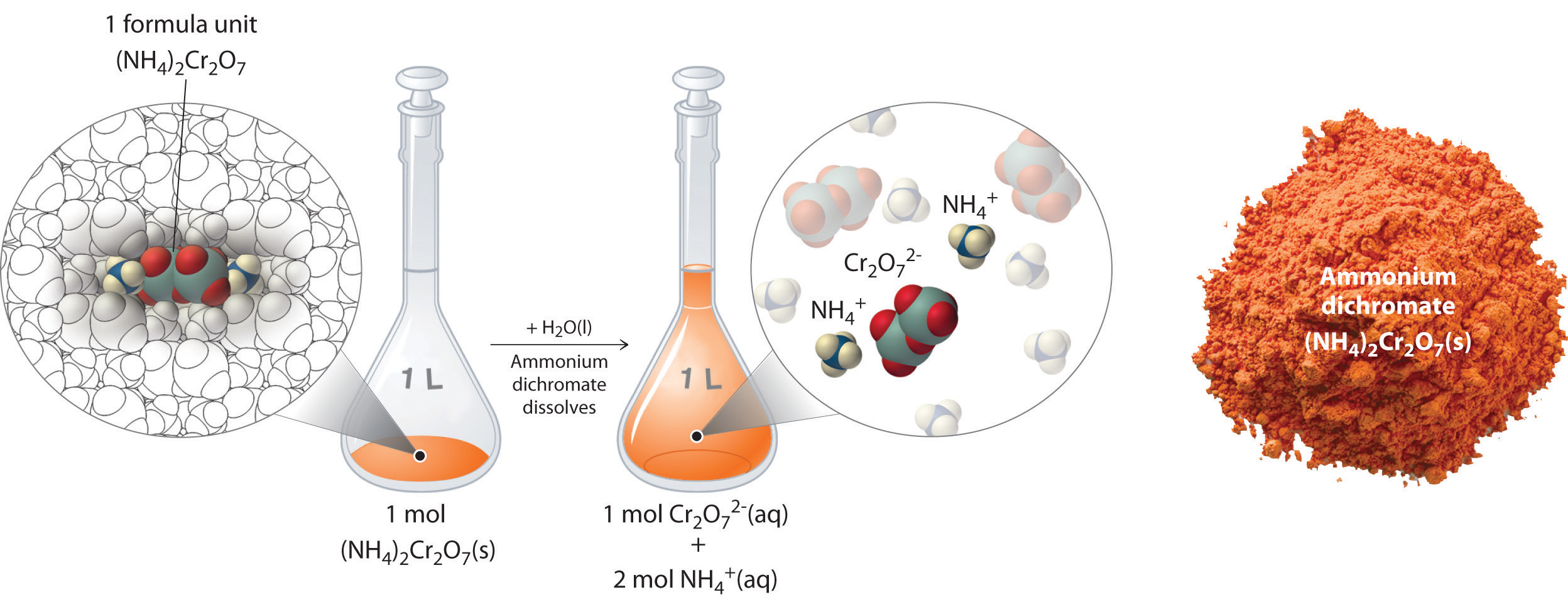
Dissolution of NaCl in Water - eduMedia If you mix two substances and the result is a homogeneous mixture, you are dealing with a solution. In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Water is a solvent. The reasons are electrostatic in nature. The cohesion of atoms and molecules derive from electrostatic links between particles ...
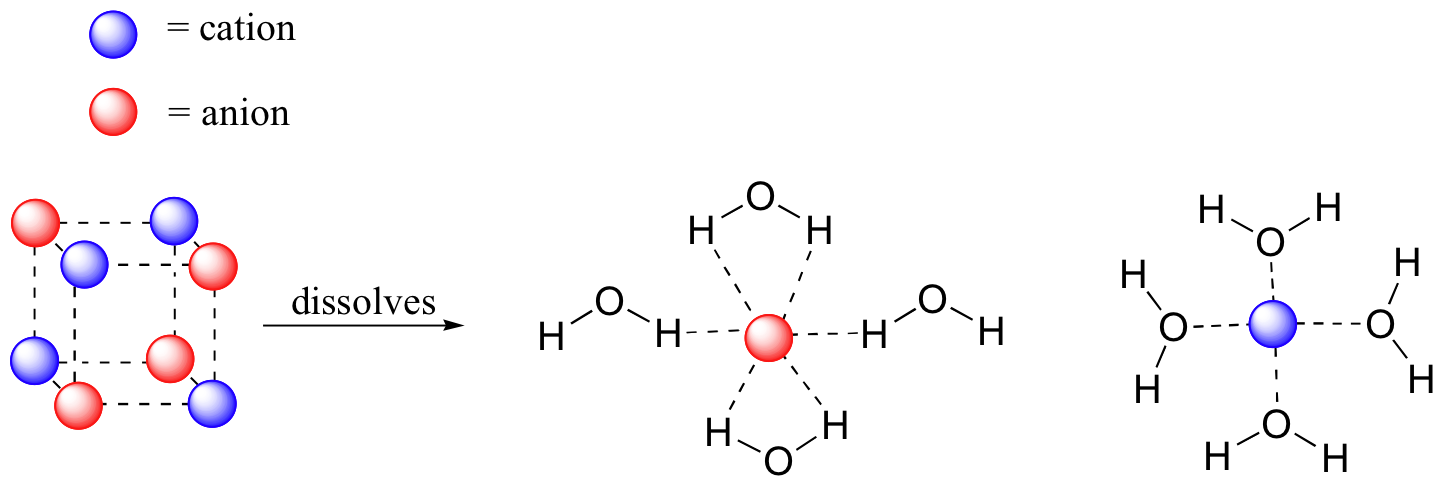
What is the formula of sodium chloride when dissolved in ... When dissolved in water, the sodium chloride framework disintegrates as the Na+ and Cl− ions become surrounded by the polar water molecules. These solutions consist of metal aquo complex with the formula [Na(H2O)8]+, with the Na-O distance of 250 pm.
Draw a diagram of table salt (NaCl) dissolved in water. | Quizlet Step 1. 1 of 2. Explanation:- \textbf {\color {#c34632}Explanation:-} Explanation:-. ⇒ \Rightarrow ⇒ Water molecules ionize NaCl molecules and convert them into positive sodium ions and negative chlorine ions. ⇒ \Rightarrow ⇒ The negative end of water molecules \textbf {The negative end of water molecules} The negative end of water ...
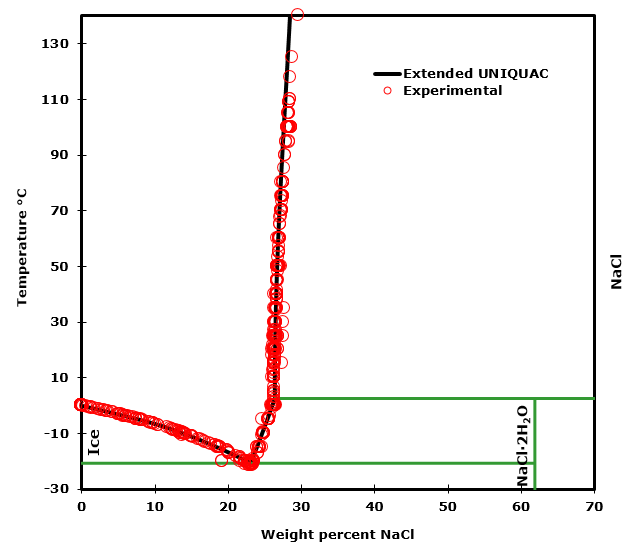
Phase Diagram of Salt Water There is an eutectic composition around 27 wt % NaCl or salt dissolved in the water. At the eutectic point, the melting or freezing temperature is as low as it ...
Solved 2. Ethanol dissolves in water. Draw a structural ... Ethanol dissolves in water. Draw a structural diagram to show the intermolecular interaction between water and ethanol. H H H H H Ethanol 3. NaCl dissolves in water. Draw structural diagrams to show the intermolecular interactions between NaCl and water. 4. Which of the following has the highest melting point? NaBr, N2, NBr3
Dissolving Salt in Water: Chemical or Physical Change? NaCl (s) → Na + (aq) + Cl - (aq) Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion). Thus, any ionic compound that is soluble in water would experience a chemical change. In contrast, dissolving a covalent compound like sugar does ...
When Nacl Dissolves In Water The Change Is Endothermic ... Why NaCl dissolves in water even though the process is endothermic? So yes, dissolving is an endothermic process because the water is gaining energy from the NaCl ionic bond or lattice, thus from the Gibbs free energy equation, G must be negative and that free energy is mostly controlled by the value of the entropy; not the enthalpy in the system.
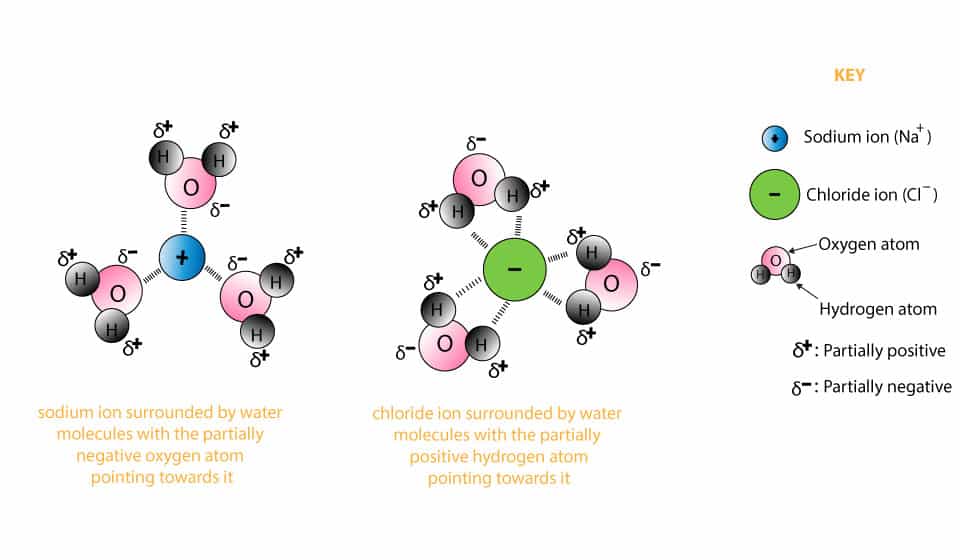
Chemistry Unit 9 - Solutions Flashcards - Quizlet Which diagram but illustrate the ion-molecule attractions that occur when the ions of NaCl(s) are added to water? A) H2O+Na and Cl+H2O B) OH2+Na and Cl+OH2 C) H2O+Na and Cl+OH2 D) OH2+Na and Cl+H2O. A. What happens when NaCl(s) is dissolved in water? A) Cl- ions are attracted to the oxygen atoms of the water. B) Cl- ions are attracted to the ...
Define dissociation. Use a diagram to explain why NaCl(s ... Maddy. Mar 29, 2021. We can draw diagrams on this site. Solid NaCl dissolves in water because the polar water molecules are attracted to the Na^+ and Cl^- and that attractions is enough to break the Na-Cl bond. Google this and you will get some good diagrams and explanations to explain just what is happening. 👍.
what occurs when nacl s is added to water - Lisbdnet.com When NaCl is added to water what happens? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a ...
What happens when sodium chloride NaCl is dissolved in water? What happens when sodium chloride NaCl is dissolved in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, […]
What happens when sodium chloride dissolves in water ... What happens when sodium chloride dissolves in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in ...
SOLVED:Draw a diagram of table salt (NaCl) dissolved in water. Video Transcript. we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. And chloride has one extra electrons in the outer plowed compared ...










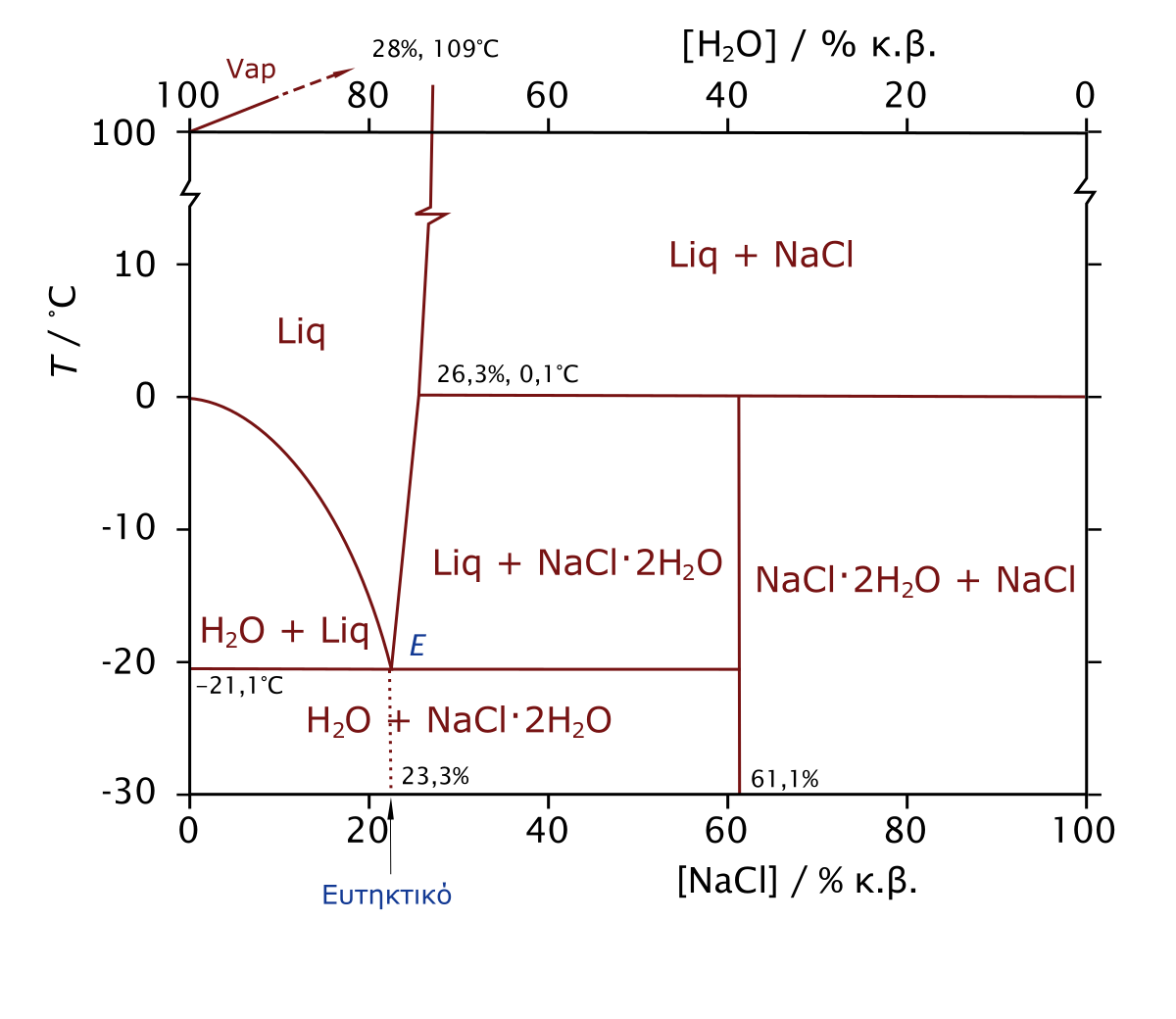





![Phase diagram of NaCl-H2O solution [21], showing the ...](https://www.researchgate.net/profile/Khadije-El-Kadi/publication/321867932/figure/fig2/AS:572547932450816@1513517472962/Phase-diagram-of-NaCl-H2O-solution-21-showing-the-reduction-in-freezing-temperature.png)



0 Response to "38 nacl dissolved in water diagram"
Post a Comment