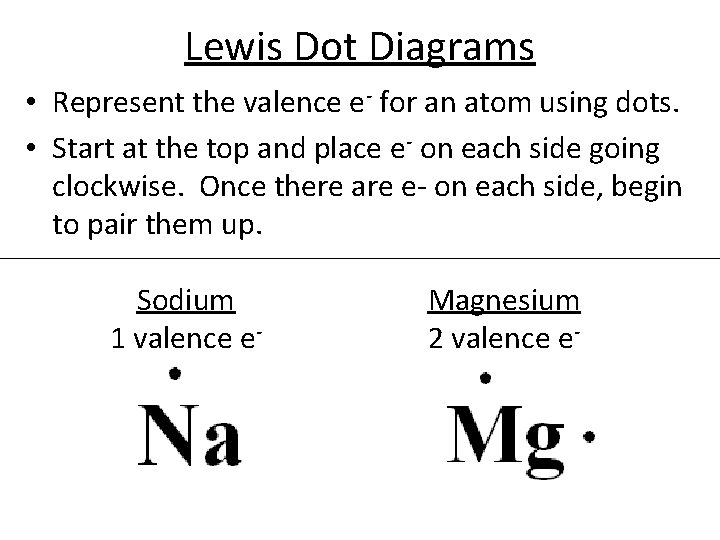
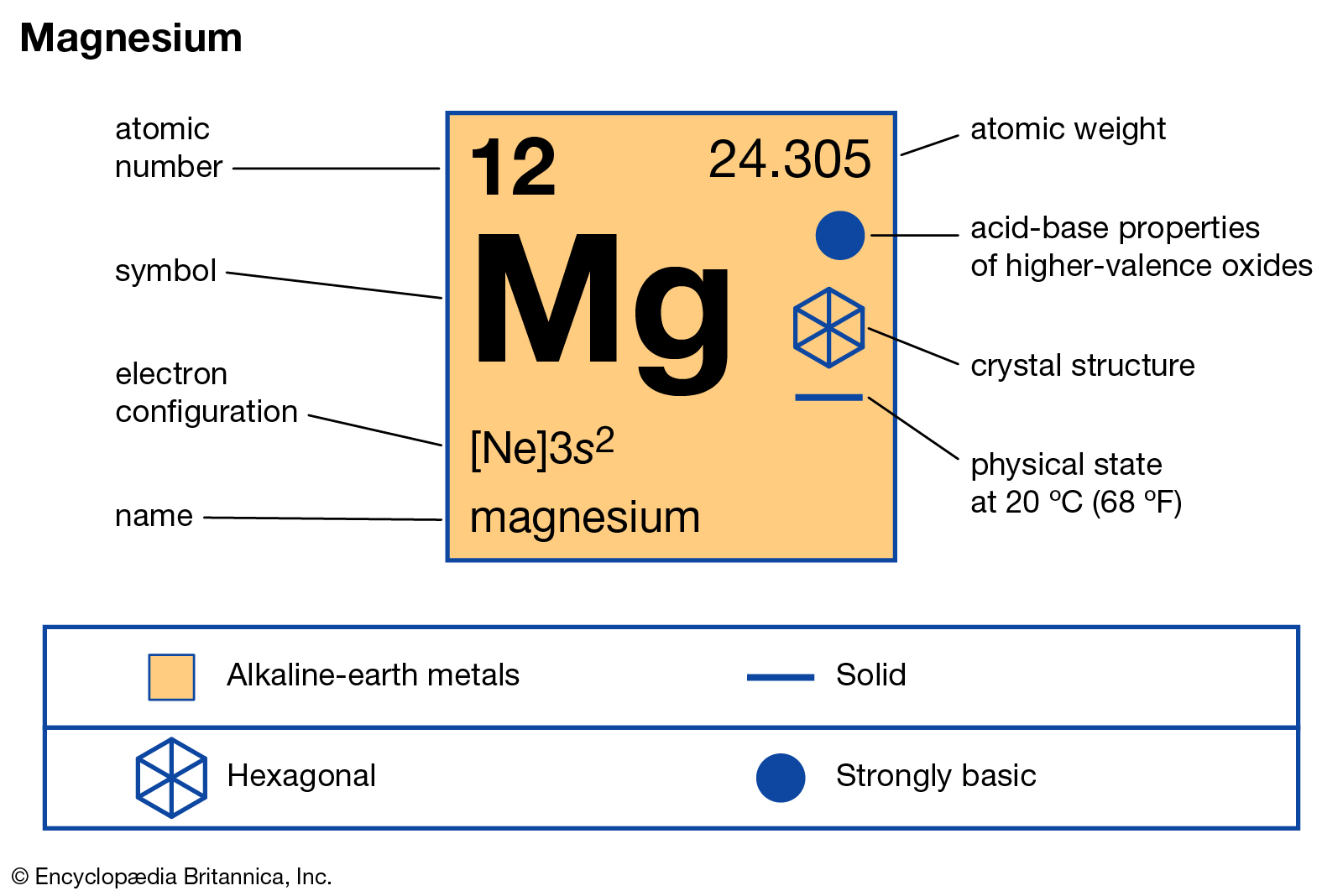
38 magnesium electron dot diagram
Electron dot diagram of magnesium oxide Solution: First examine the electron arrangement of the magnesium and nitrogen atoms. Symbol Atomic No. Bohr diagram Group No. Lewis Dots Mg 12 2 - 8 - 2 2 2 N 7 2 - 5 5 5 Write the Lewis symbols for each atom. Magnesium oxide is an ionic compound. Its formula unit (MgO) is made from one magnesium atom, which loses two electrons to become a +2 cation, and one oxygen atom, which gains those two electrons to become a -2 anion. The Lewis Structure can show the transfer of electrons from metal (low electronegativity) to non-metal (high electronegativity).
Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is ...

Magnesium electron dot diagram
Answer (1 of 4): draw a magnesium symbol and dot two dots around the symbol. Hopes this helps:) High School Chemistry/Lewis Electron Dot Diagrams ... The method explored in this lesson will be a visual representation of the valence electrons. Step 4. Search for the bond forming between the magnesium and oxygen atoms: The ionic bond will be formed between the atoms as magnesium will be donating two of its valence electrons from the 3s shell to fulfill the electron deficiency in the oxygen atom. Step 5. Now draw the Lewis structure of magnesium oxide (MgO): From the diagram, it is ...
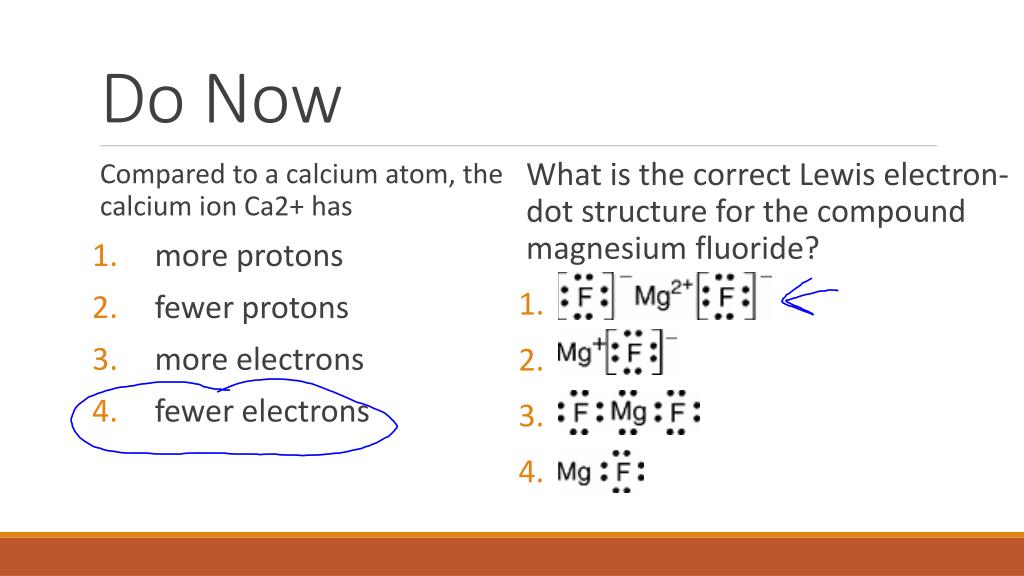
Magnesium electron dot diagram. Dec 16, 2018 · Magnesium Fluoride Lewis Dot Diagram. Magnesium fluoride is prepared from magnesium oxide with sources of hydrogen fluoride such as ammonium bifluoride. Magnesium has two electrons on its outer shell Each of the electrons will be shared with a Florine atom. This results in a compound MgF2. Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?. When magnesium donates 2 valence electrons it acquires a charge of 2+ and becomes, M g 2 + , and oxygen which gains 2 electrons acquire all the 8 valence electrons and have charge of 2-; so the electron dot structure of magnesium oxide is drawn as, Hence, the electron dot diagram for magnesium oxide consists of magnesium in M g 2 + form, and oxygen shown with 8 valence electrons (dots) with a negative charge of 2. What is the electron dot diagram for magnesium oxide? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer anor277 May 15, 2016 Well, magnesium oxide is an ionic species, which we could represent as #Mg^(2+)O^(2-)#. Explanation: Elemental ... What is the electron dot diagram for magnesium nitride? Magnesium Nitride is Mg3N2. What I think you do is draw it Mg N Mg N Mg and then draw 8 electrons around each Nitrogen so that Mg shares its ...
Draw the electron-dot structure of MgO compound and state the type of bonding. Advertisement Remove all ads. Solution Show Solution. The electron-dot structure of MgO is: Magnesium donate its two electrons to the oxygen atom to form an ionic bond in magnesium oxide, MgO. Concept: Electrovalent (or Ionic) Bond. Magnesium phosphide | Mg3P2 | CID 61546 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ... The structure should have single bond between Mg with the two bromides, each contributing one electron. The rest of the valence electrons are around bromide as to fulfill the octet rule of 8 electrons for each bromide attached to the magnesium. Step-1: MgCl2 Lewis Structure. To calculate the valence electron of each atom in MgCl2, look for its periodic group from the periodic table. The alkaline earth metal and halogen families, which are the second and 17th groups in the periodic table, are both made up of magnesium and chlorine atoms.
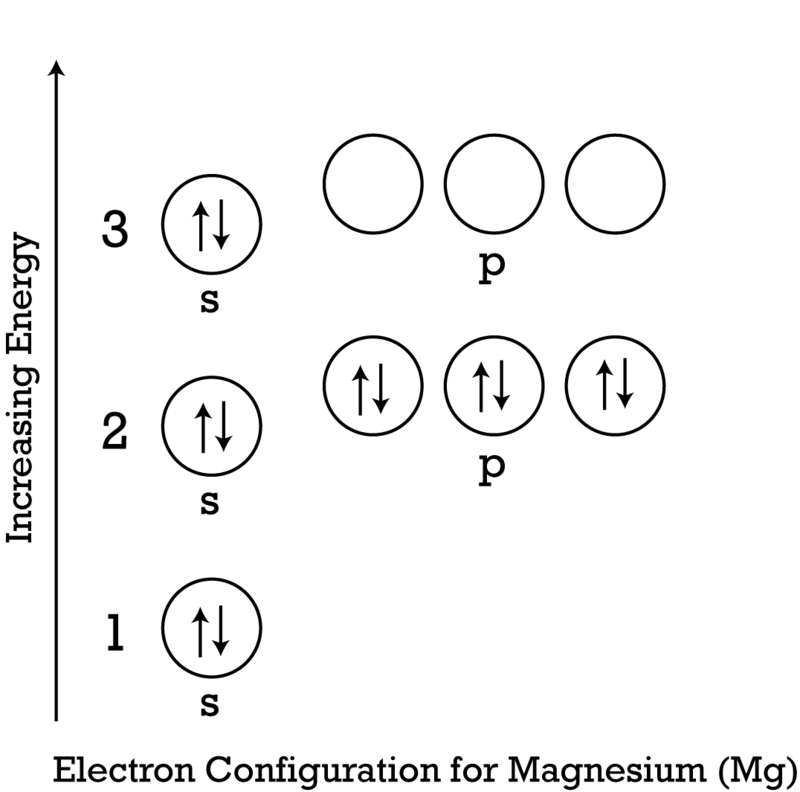
Lewis Structure of Magnesium Fluoride. To begin with, studying the Lewis structure of magnesium fluoride molecule, first, we need to study the Lewis structure of magnesium and fluorine atoms. The atomic number of magnesium is 12 where its electronic configuration is 1s2 2s2 2p6 3s2. This makes the total number of valence electrons in magnesium 2. Jan 24, 2019 · Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?.Dec 18, · Best Answer: Magnesium has 2 valence electrons and Fluorine has 7 valence electrons in order for the 2 elements to combine, you need 1 Mg atom and 2 F atoms The Mg gives one electrons to each of the F atoms, allowing the Mg to lose its 2 valence electrons and have its (new) outer energy levels fill its octet (8 electrons ... Electron dot diagram of a Magnesium atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Magnesium, we got to know, it has only 2 valence electrons. So, just represent the 2 valence electrons around the Magnesium atom as a dot. Answer: The Lewis Dot Structure for MgBr2 (Magnesium Bromide) is as follows: *P.S. sorry for my horrible writing!* The structure should have single bond between Mg with the two bromides, each contributing one electron. The rest of the valence electrons are around bromide as to fulfill the octet ...
Magnesium phosphide (Mg3P2 or P2Mg3) is IONIC because it is a combination of a metal and non-metal.Each magnesium, of which there are three, LOSES two electr...
Draw the Electron Dot Diagram and Structure of : Magnesium Chloride . CISCE ICSE Class 10. Question Papers 301. ... Question Bank Solutions 24332. Concept Notes & Videos 523. Time Tables 15. Syllabus. Advertisement Remove all ads. Draw the Electron Dot Diagram and Structure of : Magnesium Chloride - Chemistry. Advertisement Remove all ads.
Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for ... What are the valence electrons for magnesium?
[DIAGRAM] Lewis Electron Dot Diagrams Magnesium . File:Lewis dot Mg svg Wikimedia Commons . Lewis Dot Diagram For Phosphorus Wiring Diagram Source . Magnesium Fluoride Facts, Formula, Properties, Uses . P2 Lewis Structure / Chapter 5 Chemical Bonds P2 / Write .
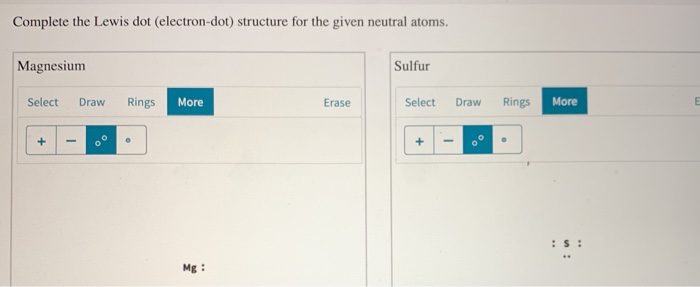
The Lewis dot structure for Magnesium is an Mg with 2 dots which stand for its two valence electrons. The Lewis dot structure for Sulfur is an S with 6 dots which stand for its six valence electrons. This is answered comprehensively here.
The electron dot structure of magnesium is given below: The electron dot structure of chlorine is given below: When 2 chlorine atoms and magnesium atoms combine by transfer of electrons then magnesium chloride is formed by transfer of electrons. Chlorine atom needs only one electron to complete its octet and magnesium cal removes two electrons ...
asked Aug 13, 2019 in Class X Science by muskan15 Expert (38.0k points) (i) Write electron-dot structures for magnesium and oxygen. (ii) Show the formation of MgO by the transfer of electrons. (iii) What are the ions present in this compound? metals and non-metals.
Draw Electron Dot Structure For The Formation Of Magnesium Oxide. Magnesium Oxide (MgO) is the most chemically active element. Its atomic number is 12, placed in 2nd group 2 and 3rd period in the modern periodic table. Formation Magnesium oxide (MgO) by the transfer of electrons. When magnesium reacts with oxygen, the magnesium atom transfers its two outermost electrons to an oxygen atom.
1. Draw an “electron dot” diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal.
"Cl" can get a noble gas s^2p^6 configuration by gaining an electron and forming a chloride ion, "Cl"^"-". The Lewis structure of "MgCl"_2 is therefore (From www.superteachertools.net) The compound is held together by electrostatic attractions between the positively charged magnesium ion and the negatively charged chloride ions.
You need to be able to draw dot-and-cross diagrams to show the ions in some common ionic The result is a sodium ion (2,8)+ and a chloride ion (2,8,8)-. Magnesium ions have the formula Mg2+, and oxide ions have the formula O2−.Electron Dot Diagram For Magnesium Chloride from electron dot diagram of chlorine, source:schematron.org lewis dot ...
A step-by-step explanation of how to draw the Lewis dot structure for Mg (Magnesium). I show you where Magnesium is on the periodic table and how to determi...
Today in this video, we will help you determine the Lewis structure for the Magnesium element. It is a group two and period three element. For determining it...
LLLL 4. Write the electron dot structure for! magnesium and chlorine. Show the formation of magnesium chloride by the transfer of I electrons.
Answer (1 of 4): draw a magnesium symbol and dot two dots around the symbol. Hopes this helps:)
electron dot diagram for Magnesium. electron dot diagram for Iodine. electron dot diagram for Boron. electron dot diagram for Sulfur. electron dot diagram for Carbon. ... Lewis structure for ONCl. 4 dots around N, double bonded to O, 2 dots around O, single bonded to Cl, 6 dots around Cl.
One magnesium atom loses two electrons, to become a +2 ion (cation).Two chlorine atoms gain one electron each to become two -1 ions (anions).These are held t...
Step 4. Search for the bond forming between the magnesium and oxygen atoms: The ionic bond will be formed between the atoms as magnesium will be donating two of its valence electrons from the 3s shell to fulfill the electron deficiency in the oxygen atom. Step 5. Now draw the Lewis structure of magnesium oxide (MgO): From the diagram, it is ...
High School Chemistry/Lewis Electron Dot Diagrams ... The method explored in this lesson will be a visual representation of the valence electrons.
Answer (1 of 4): draw a magnesium symbol and dot two dots around the symbol. Hopes this helps:)
![Answered] Show the formation of Mgo by the transfer of ...](https://hi-static.z-dn.net/files/d7b/817703aa831e0b11b0327ef2b9c06acc.png)




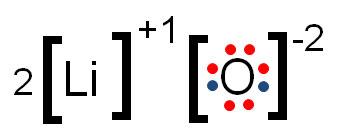
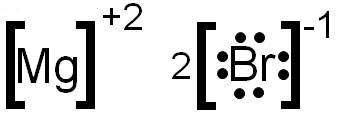










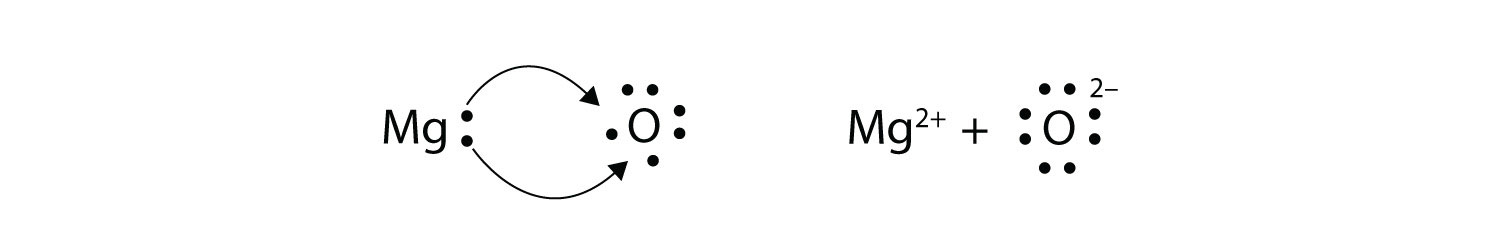





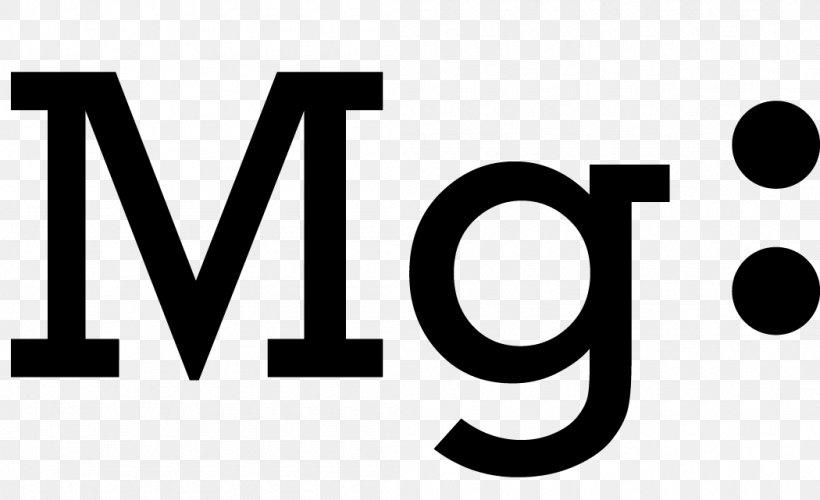
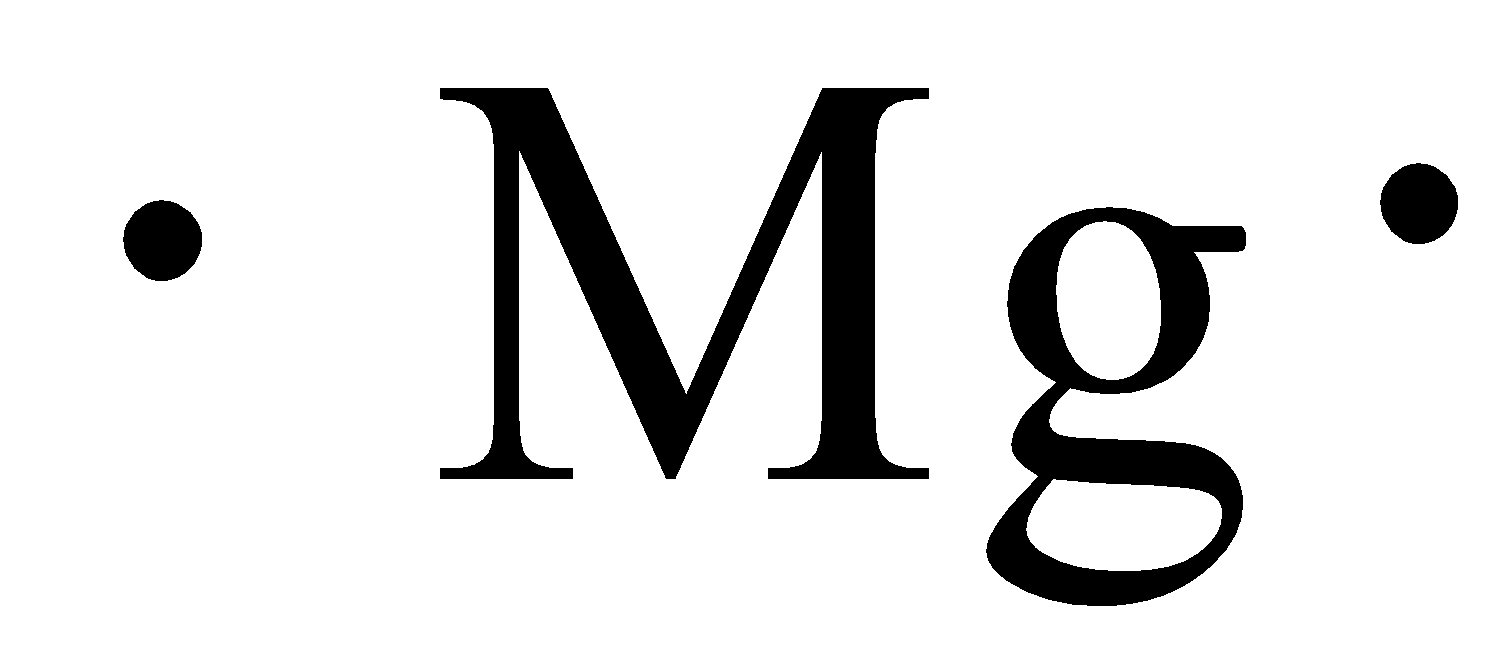


0 Response to "38 magnesium electron dot diagram"
Post a Comment