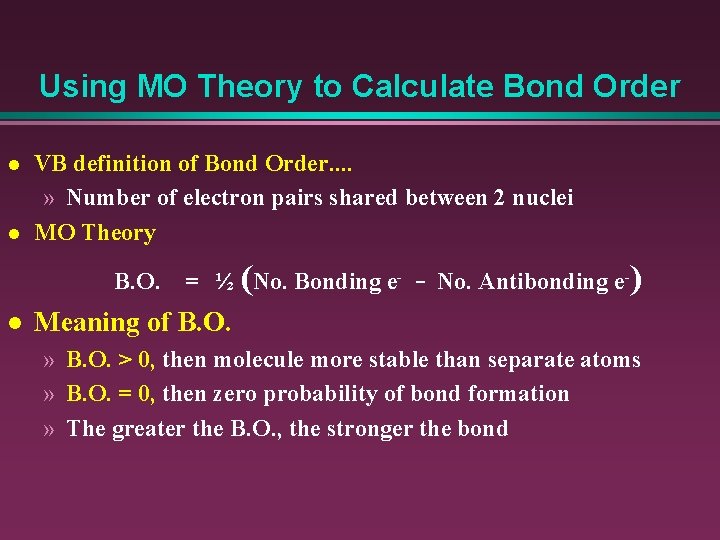
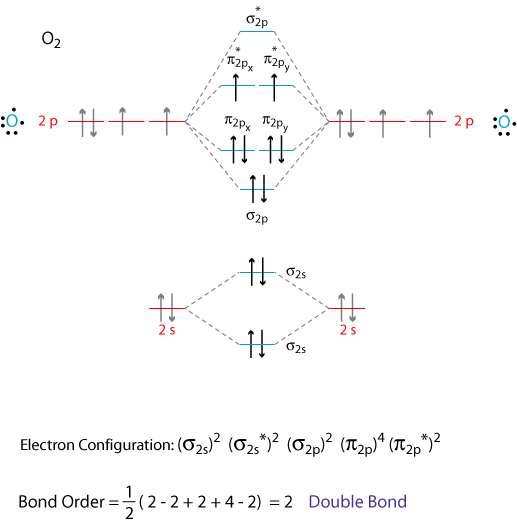
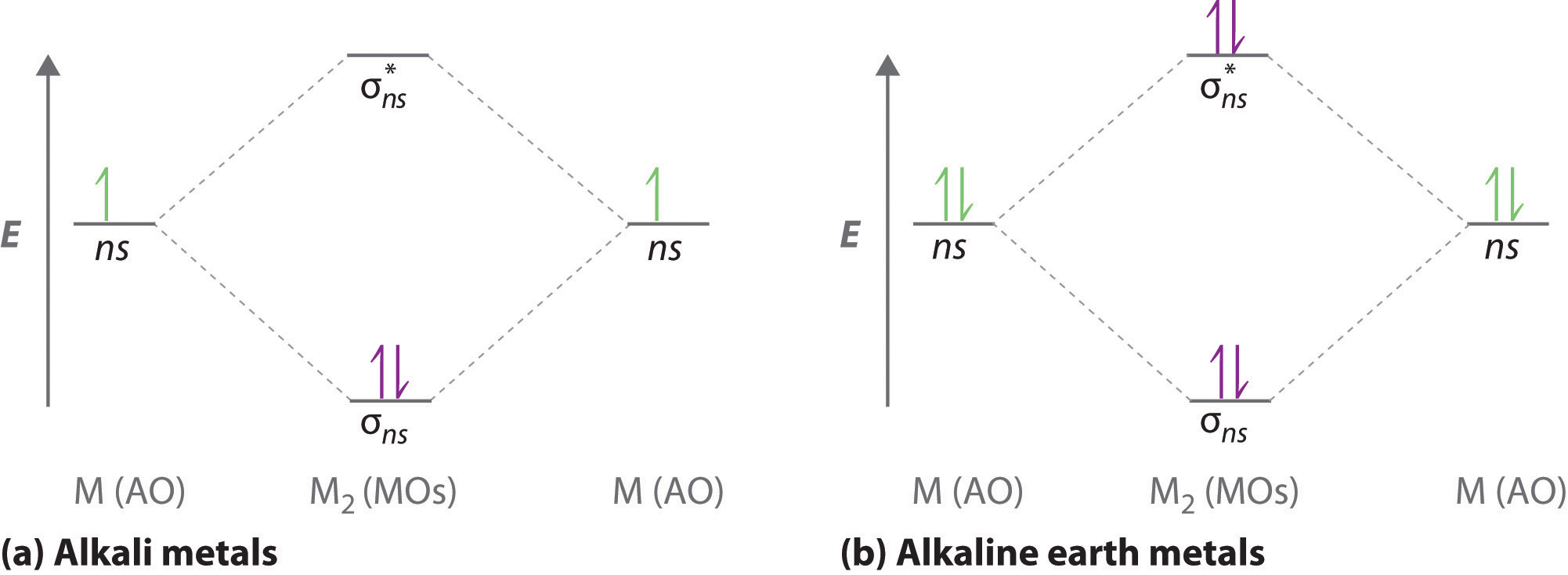
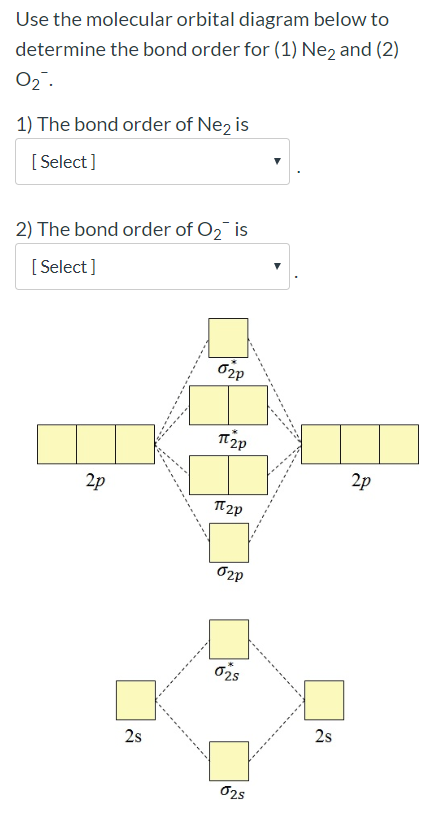
38 how to calculate bond order from mo diagram
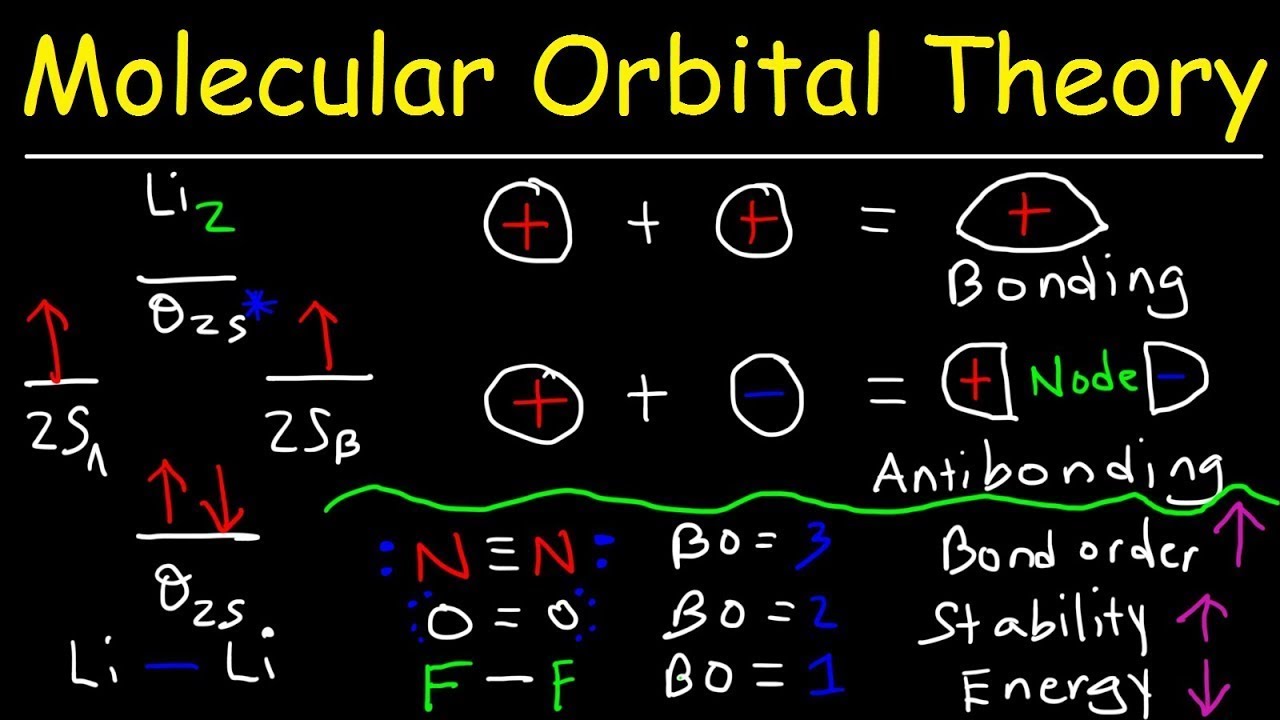
Li2 has a bond order of 1.0 (two electrons in a σ bonding orbital; see ... energy level diagram is similar to that of NO (Problem 5.7) without the.29 pages Li2 Bond Order. Here are a number of highest rated Li2 Bond Order pictures upon internet. We identified it from honorable source. Its submitted by supervision in the best field. We recognize this kind of Li2 Bond Order graphic could possibly be the most trending subject later than we allowance it in google lead or facebook.
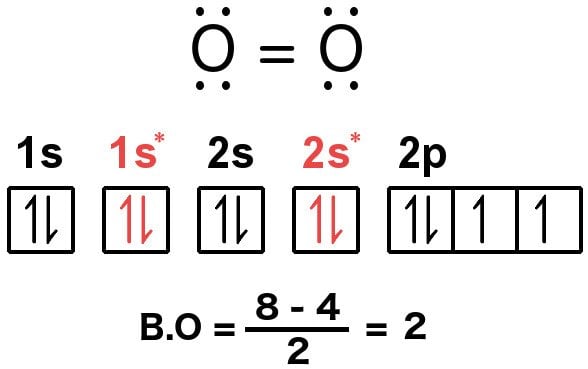
In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons. For a straightforward ...Dec 5, 2017 · Uploaded by chemistNATE
How to calculate bond order from mo diagram
*Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism) Coordination Compounds and their Biological Importance Naming Shape, Structure, Coordination Number, Ligands Biological Examples Industrial Examples *Stereochemistry *Crystal Field Theory *Molecular Orbital Theory Applied To Transition Metals Acids and Bases It follows that the equilibrium bond length, l H, is simply the average of the distribution and the bond constant, k S, can be expressed in terms of the standard deviation of that distribution. 43,92,93 43. W. Tschöp, K. Kremer, J. Batoulis, T. Bürger, and O. Hahn, " Simulation of polymer melts. I. c Dispersion diagram extracted from the 2D-FFTs of the phase mappings from 1 to 4 GHz, showing four acoustic modes in the BAW. See Supplementary Note 5 for more data. TE 1 thickness-extensional ...
How to calculate bond order from mo diagram. c Dispersion diagram extracted from the 2D-FFTs of the phase mappings from 1 to 4 GHz, showing four acoustic modes in the BAW. See Supplementary Note 5 for more data. TE 1 thickness-extensional ... It follows that the equilibrium bond length, l H, is simply the average of the distribution and the bond constant, k S, can be expressed in terms of the standard deviation of that distribution. 43,92,93 43. W. Tschöp, K. Kremer, J. Batoulis, T. Bürger, and O. Hahn, " Simulation of polymer melts. I. *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism) Coordination Compounds and their Biological Importance Naming Shape, Structure, Coordination Number, Ligands Biological Examples Industrial Examples *Stereochemistry *Crystal Field Theory *Molecular Orbital Theory Applied To Transition Metals Acids and Bases


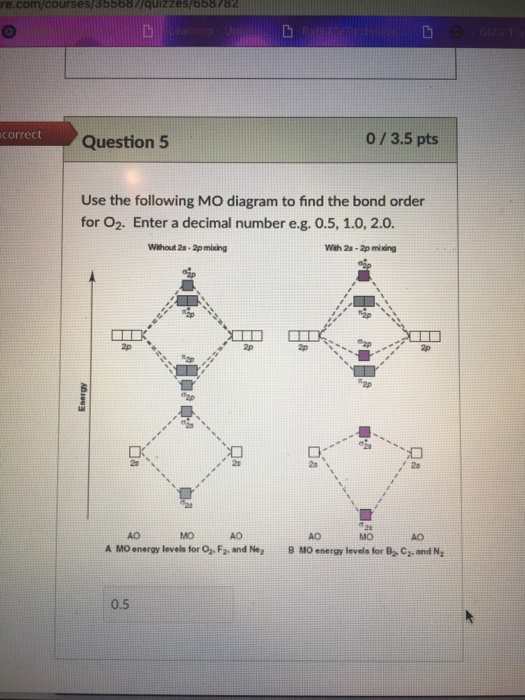

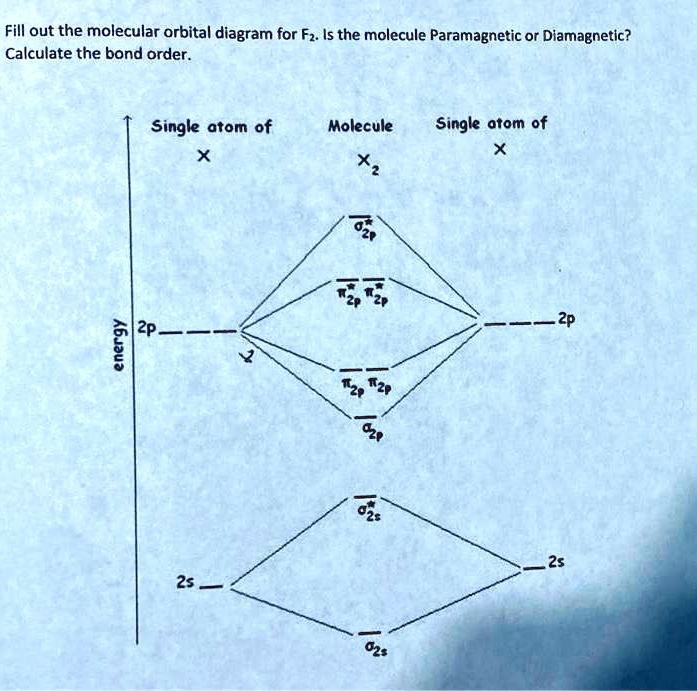
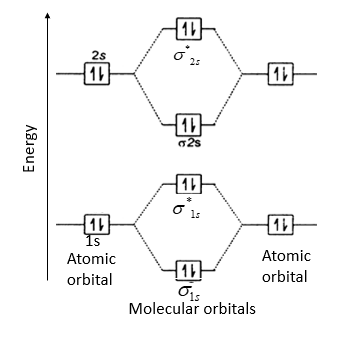

![Best Answer] draw the molecular orbital diagram of N2 and ...](https://hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)









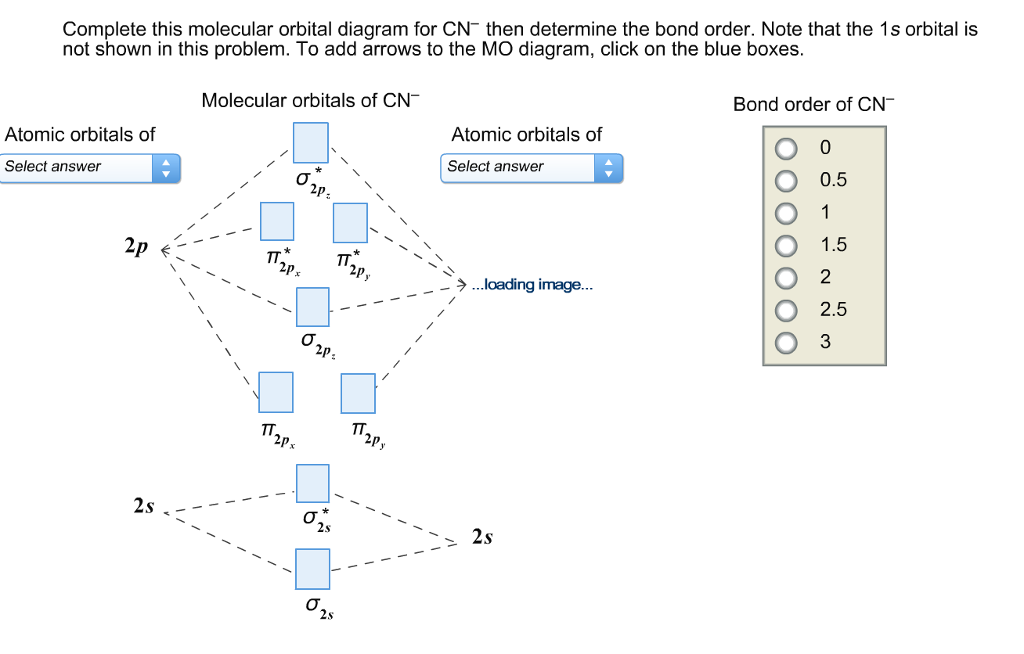


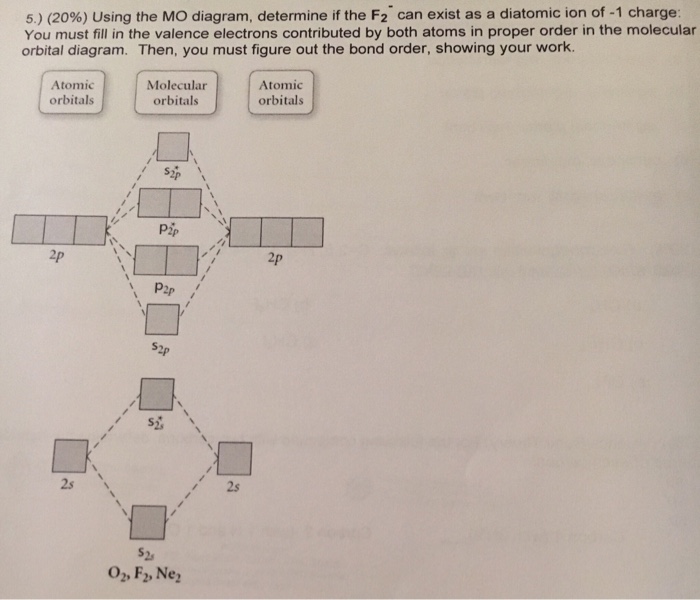

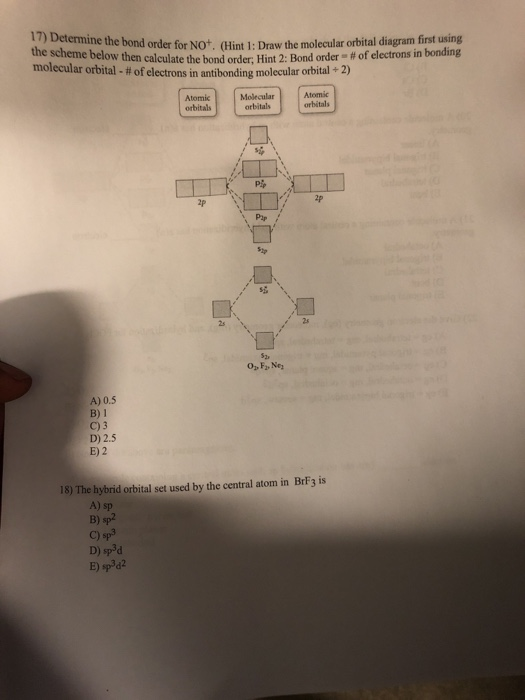



0 Response to "38 how to calculate bond order from mo diagram"
Post a Comment