39 bohr diagram for beryllium
Beryllium Bohr Model Diagram Feb 19, Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Apr 24, The isotope beryllium-9, with five neutrons, is the stable form of the atom. Creating a 3D model provides a child with a visual representation of a.
Silicon(Si) is the 14th element in the periodic table and its symbol is 'Si'. Silicon is a semiconductor material. The electron configuration of silicon and the orbital diagram is the main topic of this article. Also, valency and valence electrons of silicon, various reactions, and compound formation, bond formation have been discussed ...
According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons.
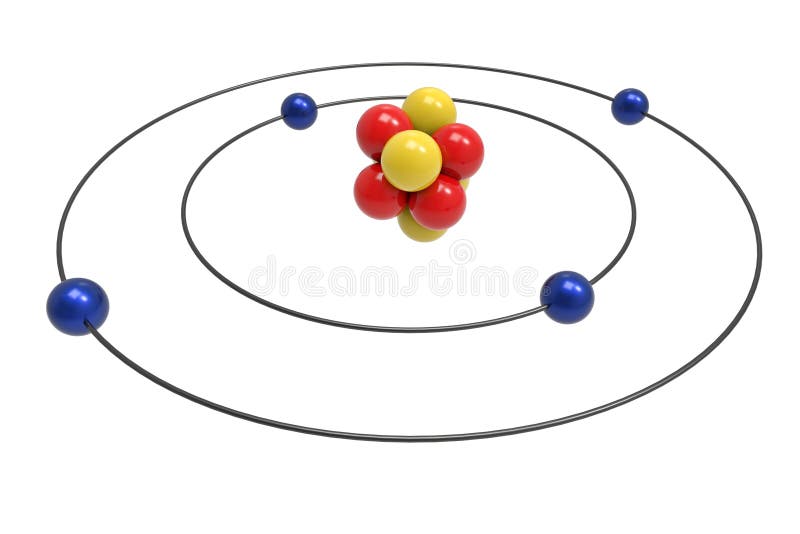
Bohr diagram for beryllium
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
In atomic physics, the Rutherford-Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , is a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of.draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for beryllium. draw a Bohr ...
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Bohr diagram for beryllium.
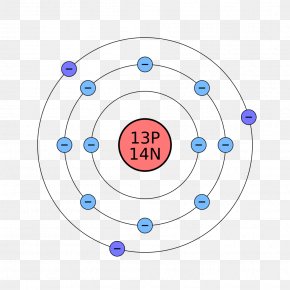
Beryllium Bohr Diagram Draw Bohr Rutherford Diagrams For The Most Common Isotope Of Each Of. Beryllium Bohr Diagram Fileelectron Shell 004 Beryllium No Labelsvg Wikimedia Commons. Beryllium Bohr Diagram Lithium Atom Bohr Model Proton Neutron Electron Illustration Stock.
Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ...
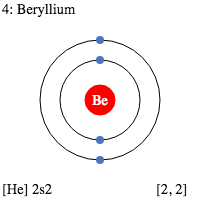
Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ...
diagram wiring diagram database bohr, beryllium bohr diagram drawing, beryllium chloride wikipedia, electron arrangement of the first 20 elements pass my exams, the structure of an atom explained with a labeled diagram, atom diagram universe today, how to represent electrons in an energy level diagram, beryllium atom structure grand unified theory,
Bohr Model Worksheet With Answers Fill Online Printable Bohr Model Physical Science Middle School Worksheets. The following drawings are bohr models for a beryllium fluorine and carbon atom. Bohr Model Diagram Cards Bohr Model Super Teacher Worksheets Teaching Chemistry All things algebra answer key unit 8.Bohr model drawing worksheet answer key.
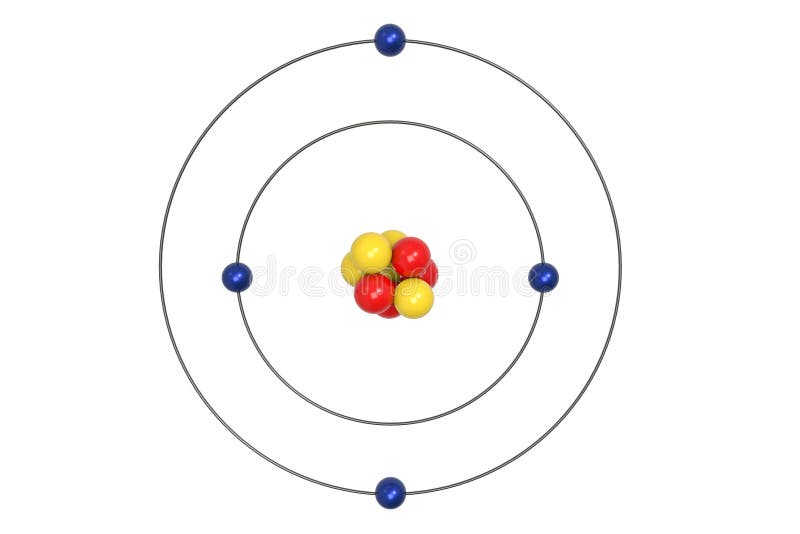
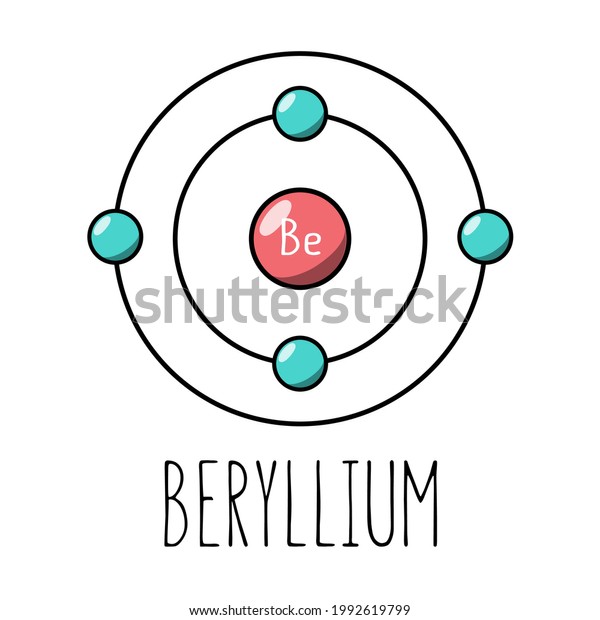
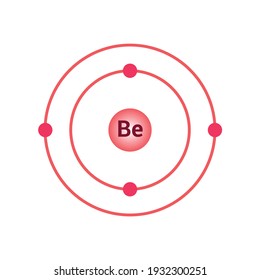
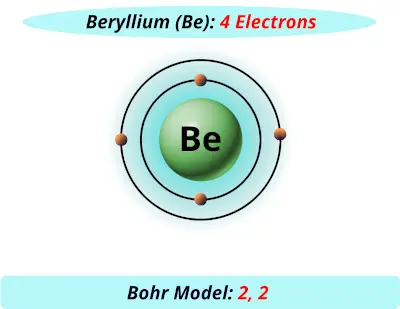
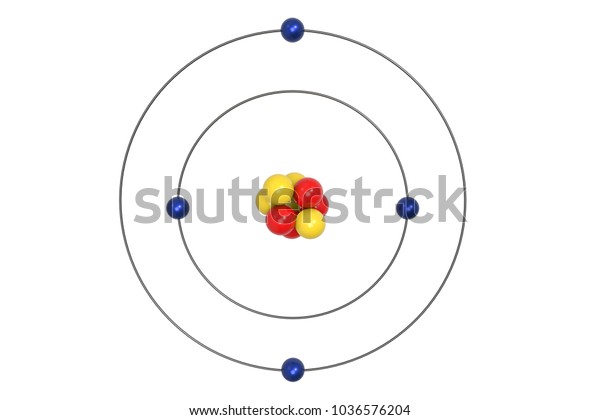
What is beryllium Bohr diagram? The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. ... (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons. The 4 electrons are arranged with 2 electrons in the first orbital and 2 electrons in the second orbital.
What is beryllium Bohr diagram? The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons. The 4 electrons are arranged with 2 electrons in the first orbital and 2 electrons in the second orbital.
The phosphorus bohr model has 3 shells because it has 15protons and 16 neutrons. Beryllium Atom Bohr model with proton, neutron and electron. 3d illustration ( is about 31, so 15protons = 16 neutrons the number of electrons is the same number of protons in an. Bohr Model Diagrams. It is the original image provided by the contributor.
To draw the Bohr model of an atom, we should follow 4 or 5 basic steps. Find the number of protons, electrons, and neutrons of an atom. Draw the nucleus of an atom. Write the number of protons and neutrons at the center of the nucleus. Draw the first electron shell and put the electrons as a dot in it. Draw the second electron shell, third ...
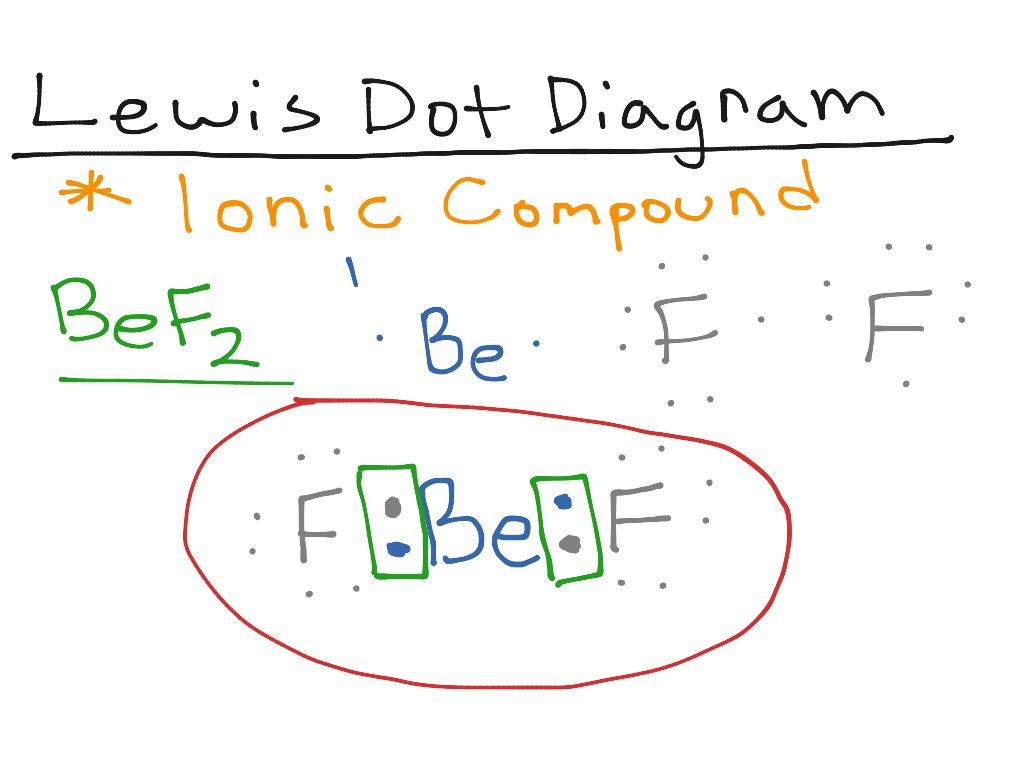
Answer to Class Example - Bohr Electron Configuration Drawing for Beryllium after it has satisfied the Octet Rule in Lesson 1.4
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
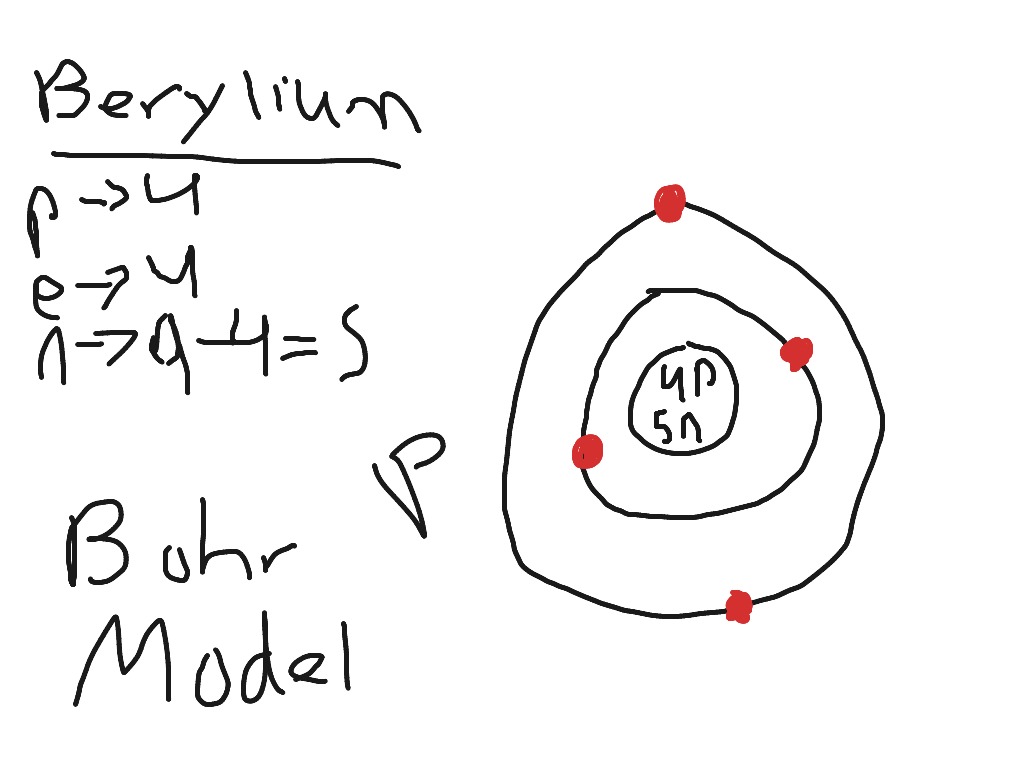
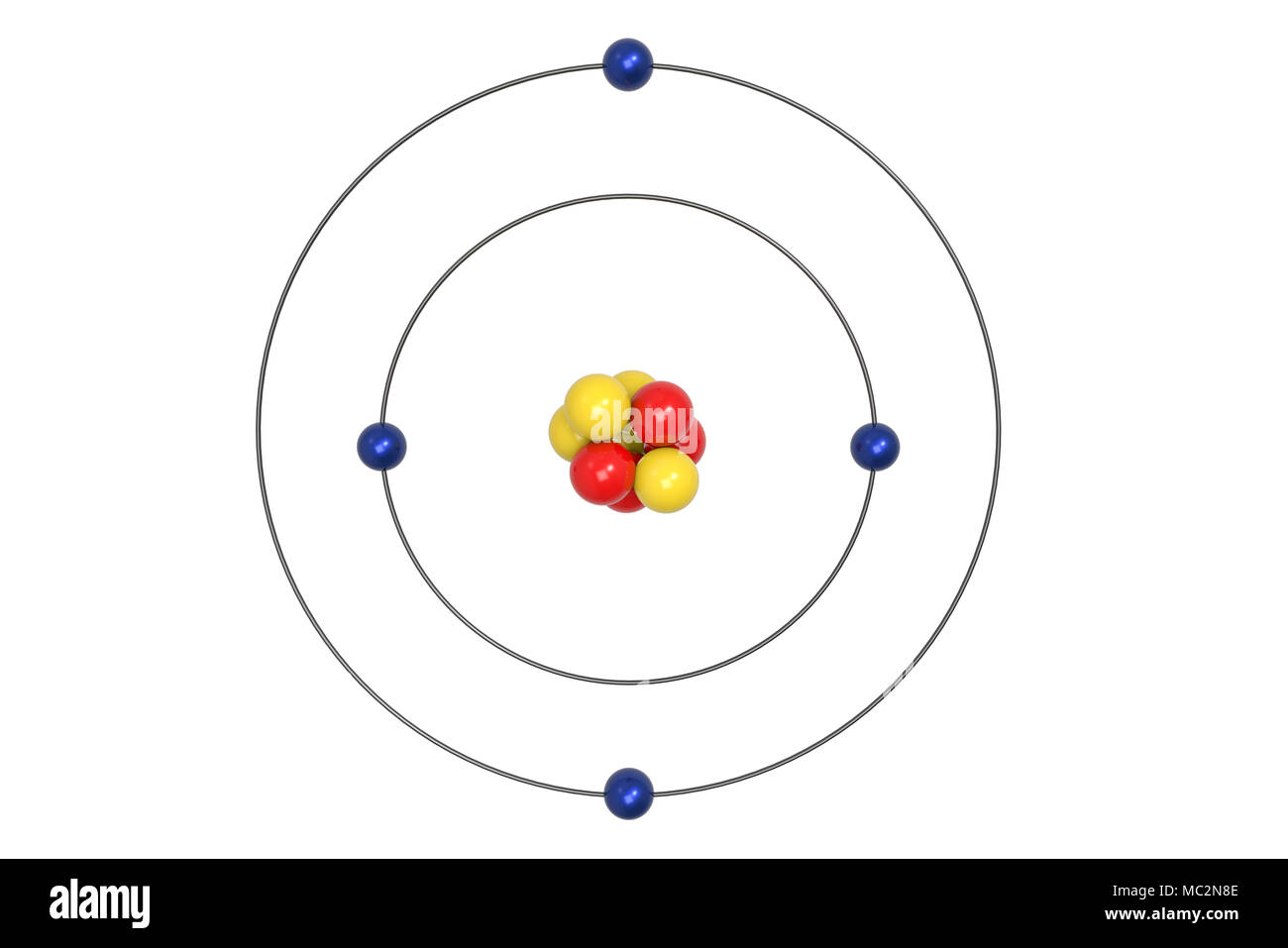
Sep 27, 2018 · Bohr Diagrams of Atoms and Ions. Bohr Model Diagrams. 1. Beryllium –. P- 4 protons. E- 4 electrons. N- 5 neutrons. 2. Sodium –. P- 11 protons. Draw a Bohr Model of Beryllium Draw a Bohr Model of Chlorine Activity. E- 11 electrons. N- 12 neutrons.Name Period Date Bohr Model Diagrams. Use the information provided for each element to draw Bohr Model diagrams.
Beryllium 2. Hydrogen 009 5. Sodium 1 8. Magnesium 11. Silicon . Science 9 Chapter 2 — Elements and the Periodic Table Identify the elements whose Bohr model diagrams are shown below. Write the names of the elements in the spaces provided. O O (f) (a) oo (c) O (d) (b) (c) (d) Name Use with textbook pages 64-67.
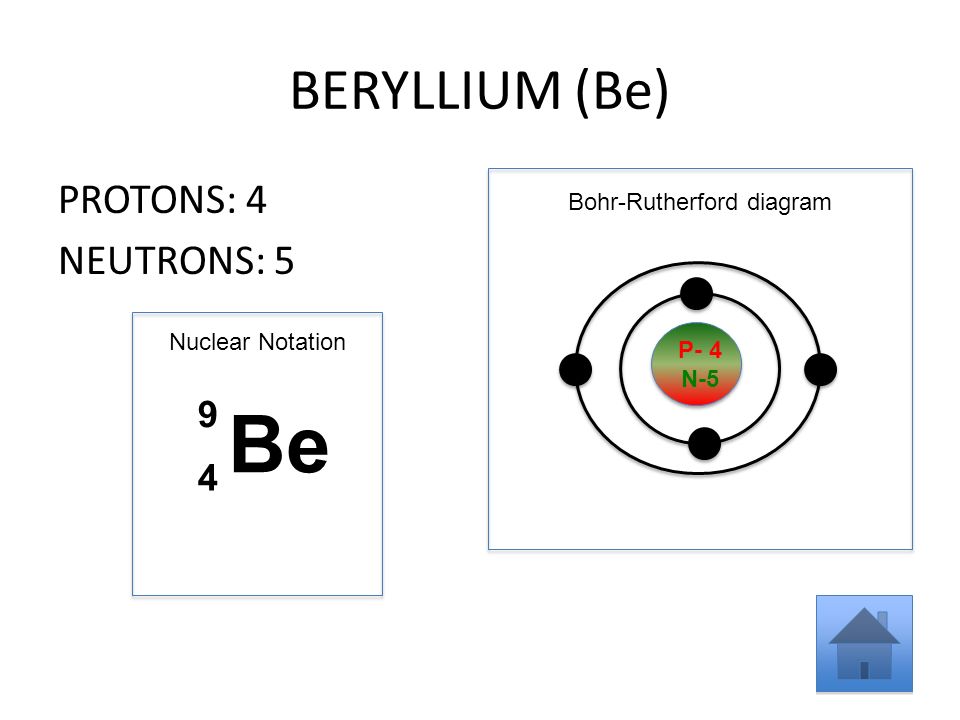
Name: Beryllium Symbol: Be Atomic Number: 4 Atomic Mass: 9.012182 amu Melting Point: 1278.0 °C (1551.15 K, 2332.4 °F) Boiling Point: 2970.0 °C (3243.15 K, 5378.0 °F) Number of Protons/Electrons: 4 Number of Neutrons: 5 Classification: Alkaline Earth Crystal Structure: Hexagonal Density @ 293 K: 1.8477 g/cm 3 Color: gray Atomic Structure
Learn bohr diagrams with free interactive flashcards. Choose from 221 different sets of bohr diagrams flashcards on Quizlet.
Beryllium oxide is a beryllium molecular entity consisting of beryllium (+2 oxidation state) and oxide in the ratio 1:1. In the solid state, BeO adopts the hexagonal wurtzite structure form while in the vapour phase, it is present as discrete diatomic covalent molecules. It has a role as a carcinogenic agent.
What is the pattern for the first 4 she…. Shows how many electrons each shell has. Niels Bohr. The number of protons and neutrons are shown in the centre and…. 2, 8, 8, 18. Bohr Diagram. Shows how many electrons each shell has. Named after. Niels Bohr.
File:electron Shell 004 Beryllium. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. The isotope beryllium-9, with five neutrons, is the stable form of the atom. drawing electron configurations. Creating a 3D model provides a child with a visual ...
Bohr Model Diagrams and Lewis Dot Structures. Use the information provided for each element to draw Bohr Model diagrams. Rather than drawing individual protons and neutrons, you may simply label how many of each there are in the nucleus (e.g. He: 2p, 2n).
Oct 28, 2018 · 1. Beryllium – P. E. N 2. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.
Try to make a Bohr-Rutherford ion for phosphorous. 31 P 15 3-Metals will often form bonds with non metals. This is the Basis for Ionic compounds ... Snc2d Bohr ions diagrams copy Created Date: 9/16/2015 12:45:23 AM ...
The Bohr Model for Beryllium (Be) has 4 protons in the nucleus due to the atomic number of Be being 4. The Mass number is 9 which means Beryllium needs 5 neutrons in the nucleus. (Mass number = protons + neutrons, 9 = 4 + n). Beryllium has four electrons to balance the four protons.
According to the Bohr diagram of Beryllium, the outer shell is L-shell which contains 2 valence electrons. Properties of Beryllium It has a hexagonal closed packed crystal structure.' It has a boiling point of 2469 °C and a melting point of 1287 °C. It appears as white-gray metallic and lightweight in nature.




















0 Response to "39 bohr diagram for beryllium"
Post a Comment