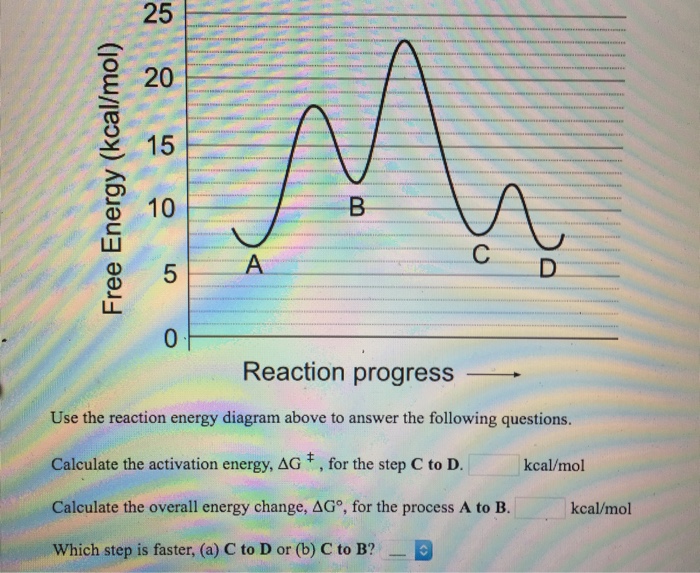
41 use the energy diagram for the reaction to answer the questions.
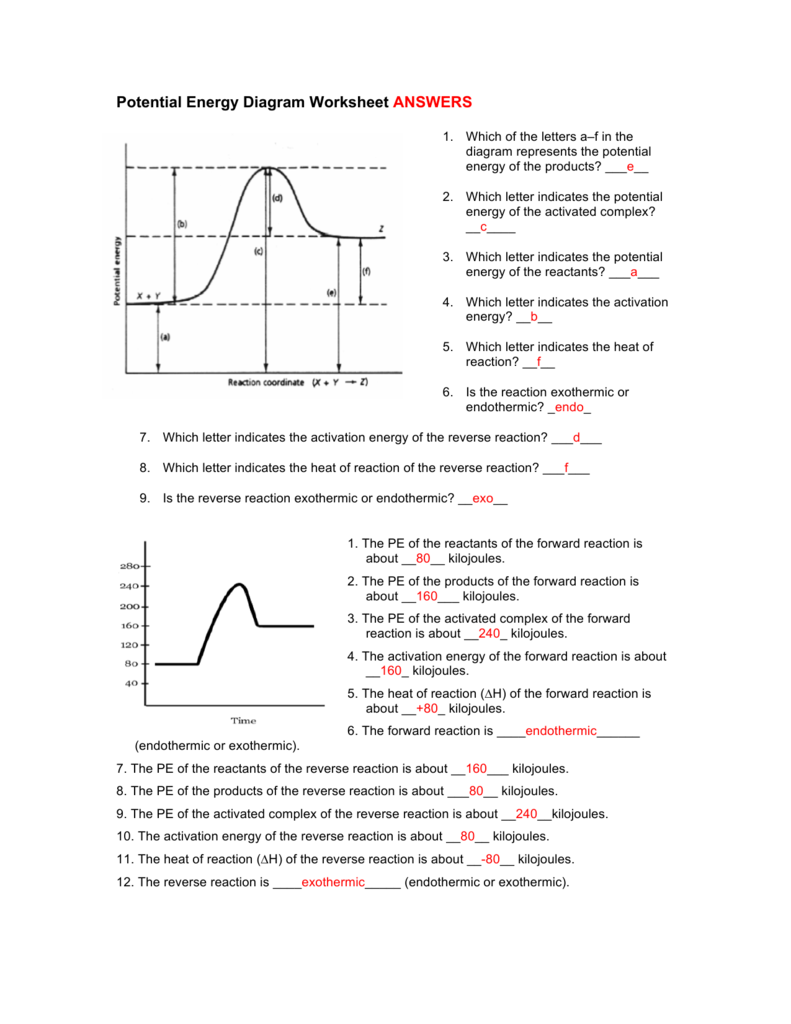
Use the reaction energy diagram above to answer the following questions. For the reverse reaction on the graph above. Calculate the activation energy g for the step c to b kcalmol calculate the overall energy change g for the process b to d kcalmol which step is faster a b to a or b a to b. The arrow labeled c represents a transfer of chemical ... Use this energy diagram to answer these questions. 1. The heat content of the reactants of the forward reaction is about kilojoules. 2. The heat content of the products of the forward reaction is about kilojoules. 3. The heat content of the activated complex of the forward reaction is about kilojoules. 4.
Q. How much energy is released when two moles of methane (CH 4) reacts at 298 K and 101.3 kPa according to the following equation: CH 4 (g) + 2O 2 (g) → CO 2 (g) + 2H 2 O(l)
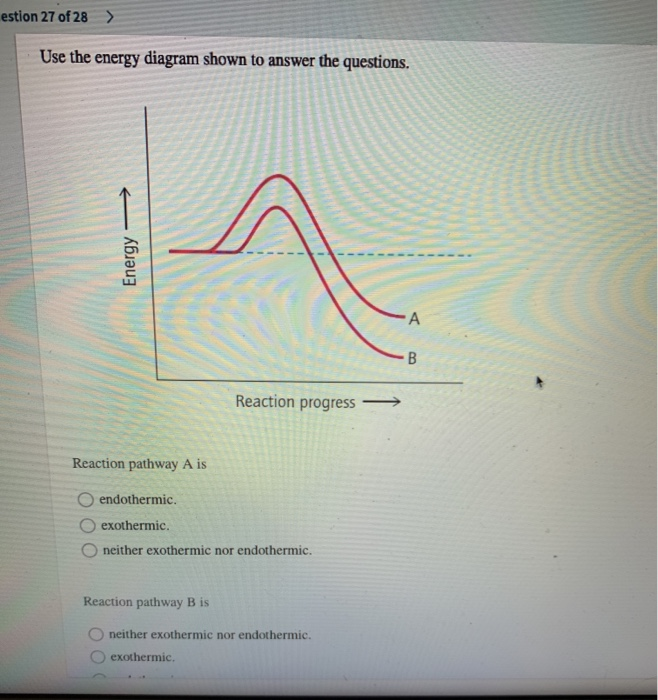
Use the energy diagram for the reaction to answer the questions.
Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? How many intermediates are there in the reaction? Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Assume the frequency factor (?) is the same for each elementary reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right. The components of a one-step energy diagram are: Chemistry questions and answers; Energy Diagram - Self-Check: (all questions apply to the energy diagram shown) Energy Reaction Progress 1. How many intermediates are shown on the energy diagram? 2. How many transition states are shown? 3. Which step has the highest activation barrier? 4. Which step has the lowest activation barrier? 5.
Use the energy diagram for the reaction to answer the questions.. Use the reaction energy diagram below to answer the following questions. (8 pts) Energy D Reaction Progress I a. The transition state is found at on the diagram. b. The products are found at on the diagram c. The free-energy change for the reaction is indicated at on the diagram. d. The reactants are found at on the diagram 7. Chemistry questions and answers. 6) Use the Energy diagrams of two reactions to answer the following: Activation energy Activation energy Products Reactants Products Reactants Progress of reaction Progress of reaction Reaction 1 Reaction II A) Which reaction is likely to be faster? Explain. B) Which of the reactions would release energy? Explain. Answer to: Here is an energy diagram for the reaction: Use the energy diagram to answer these questions. Can you determine the activation energy of the reverse. Subjects . Science Chemistry Video Lessons Exam Reviews ACS Video Solutions Solutions Library. Potential energy. Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in the reaction? transition states: B How many intermediates are there in the reaction? A D Reaction progress intermediates: Which step of the reaction is the fastest?
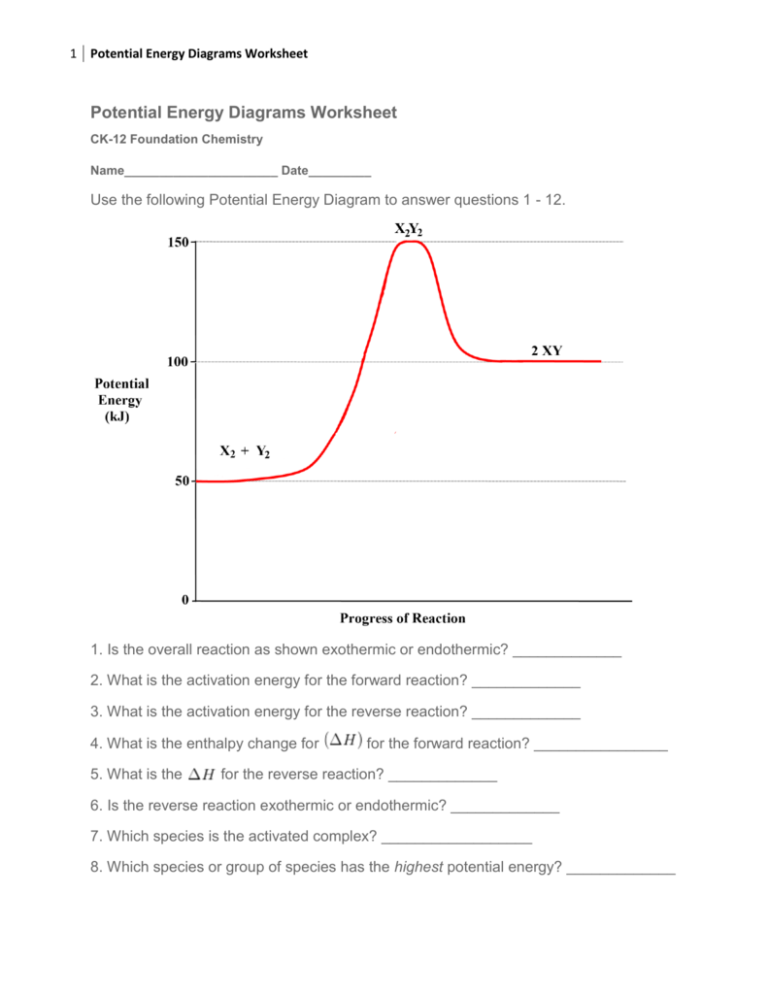
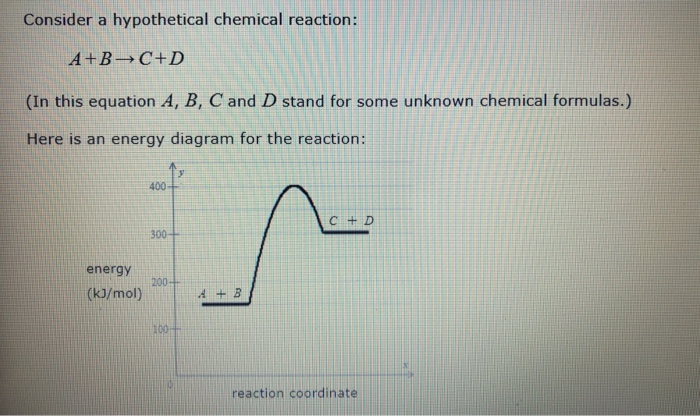
Date: Potential Energy Diagrams Graph 3: Use the potential energy diagram for the reaction A + B C + D to answer the following questions. Is the reaction exothermic or D to answer Use the energy diagram for the reaction A the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction ... Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant?
Use the energy diagram for the reaction A → D to answer the questions. Q. Which of the following has ΔG°f = 0 at 25°C?a. O3 (g)b. O (g)c. H2O (g)d. H2O (l)e. Na (s) Q. Predict the sign of ΔG for an endothermic reaction with a decrease in entropy. Use this energy diagram to answer these questions. 1. The enthalpy of the reactants of the reaction is about kilojoules. 2. The enthalpy of the products of the reaction is about kilojoules. 3. The activation energy of the reaction is about kilojoules. 4. The heat of reaction (ΔH) of the reaction is about kilojoules. Science; Chemistry; Chemistry questions and answers; Use the reaction energy diagram below to answer the following question(s). i) The reaction depicted in this reaction energy diagram can best be described as: a. a slow exothermic reaction b. a fast exothermic reaction c. a slow endothermic reaction d. a fast endothermic reaction ii) The transition state is found at point _____ on the diagram ii) Use the reaction energy diagram above to answer the following questions. Use the reaction energy diagram above to answer the following questions. Explain why this is true or false. A the diagram below at right shows the energy pathway for the reaction o3 no no2 o2. The arrow labeled c represents a transfer of chemical energy to mechanical energy.
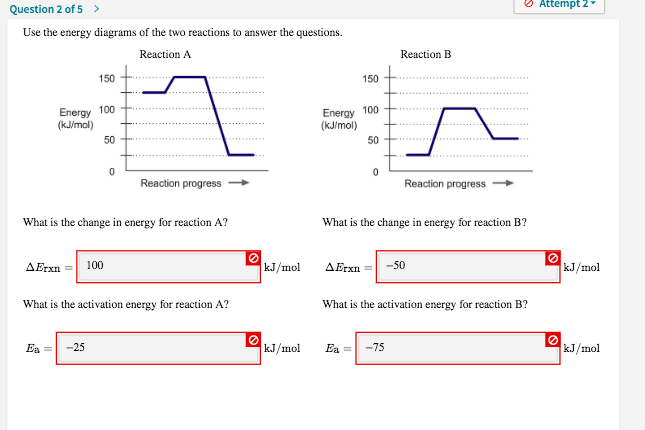
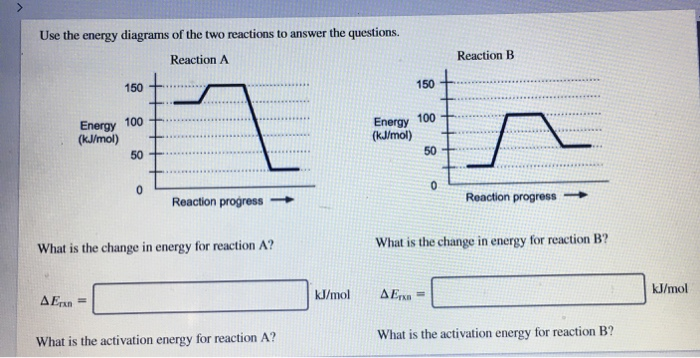
Chemistry questions and answers; Use the energy diagrams of the two reactions to answer the questions. Reaction A Reaction B 150+ Energy 100 (kJ/mol) HH Energy (kJ/mol) 50 TH +..... Reaction progress Reaction progress- What is the change in energy for reaction A?
Chemistry questions and answers; Energy Diagram - Self-Check: (all questions apply to the energy diagram shown) Energy Reaction Progress 1. How many intermediates are shown on the energy diagram? 2. How many transition states are shown? 3. Which step has the highest activation barrier? 4. Which step has the lowest activation barrier? 5.

3 Please Answer The Following Questions Concerning The Two Energy Diagrams Depicted Below 10 Pts Energy Homeworklib
Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right. The components of a one-step energy diagram are:

Match The Reaction To Its Energy Diagram Assume That Each Reaction Is An Overall Exothermic Process I E The Products Have Lower Potential Energy Than The Starting Materials Study Com
Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? How many intermediates are there in the reaction? Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Assume the frequency factor (?) is the same for each elementary reaction.

A Does This Graph Represent An Endothermic Or An Exothermic Reaction Explain Your Answer B What Brainly Com
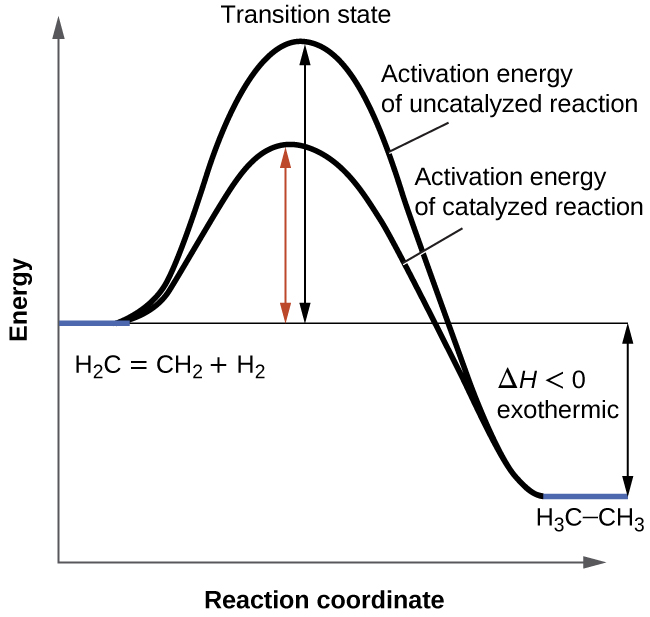
How Can I Draw A Simple Energy Profile For An Exothermic Reaction In Which 100 Kj Mol 1 Is Evolved And Which Has An Activation Energy Of 50 Kjmol 1 Socratic
Solved 10 Shown Below Is A Reaction Energy Diagram Answer The Questions Below Using This Diagram Nrg Products Reactants Reaction Pathway A Is T Course Hero
Solved 25 20 15 Free Energy Kcal Mol 10 B 5 A C O Reaction Progress Use The Reaction Energy Diagram Above To Answer The Following Questions Calc Course Hero

How Can I Draw A Simple Energy Profile For An Exothermic Reaction In Which 100 Kj Mol 1 Is Evolved And Which Has An Activation Energy Of 50 Kjmol 1 Socratic

Which Of The Following Energy Diagrams Is Of A Reaction With One Transition State Which Of The Following Energy Diagrams Is Of A Reaction With One Intermediate Image Src Reaction39466578977983968 Study Com

Use The Graph And Diagram Below To Answer The Question Part A Explain How Enzyme A Acts As A Brainly Com

Worksheet 1 Docx Worksheet It Is Time To Practice Using Potential Energy Diagrams Respond To The Three Questions Below On Energy Diagrams And Submit Course Hero
Solved Use Your Knowledge Of The Collision Theory And Use The Potential Energy Diagram Given To Answer Questions 1 Through 5 Which Letter Represen Course Hero















0 Response to "41 use the energy diagram for the reaction to answer the questions."
Post a Comment