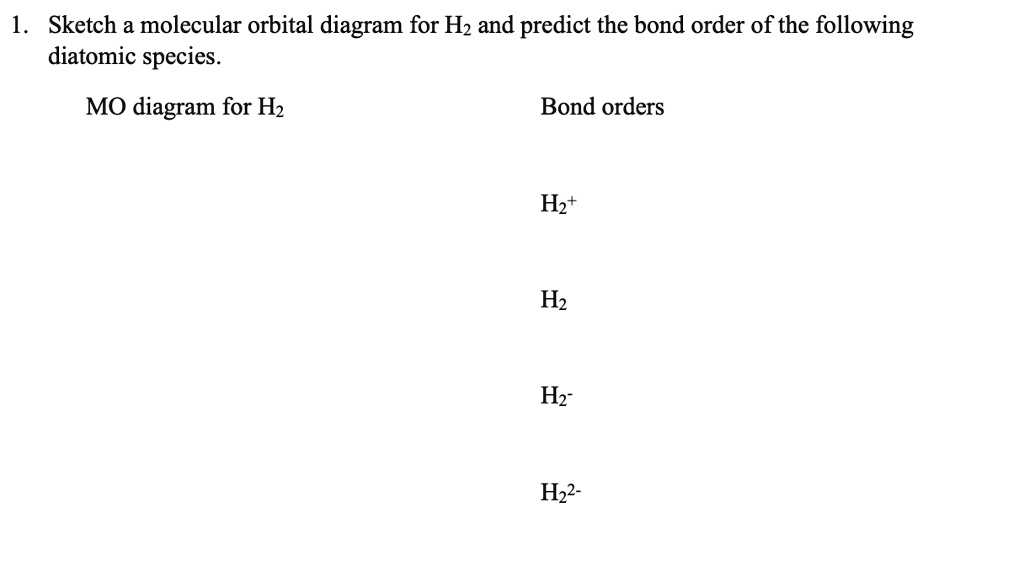
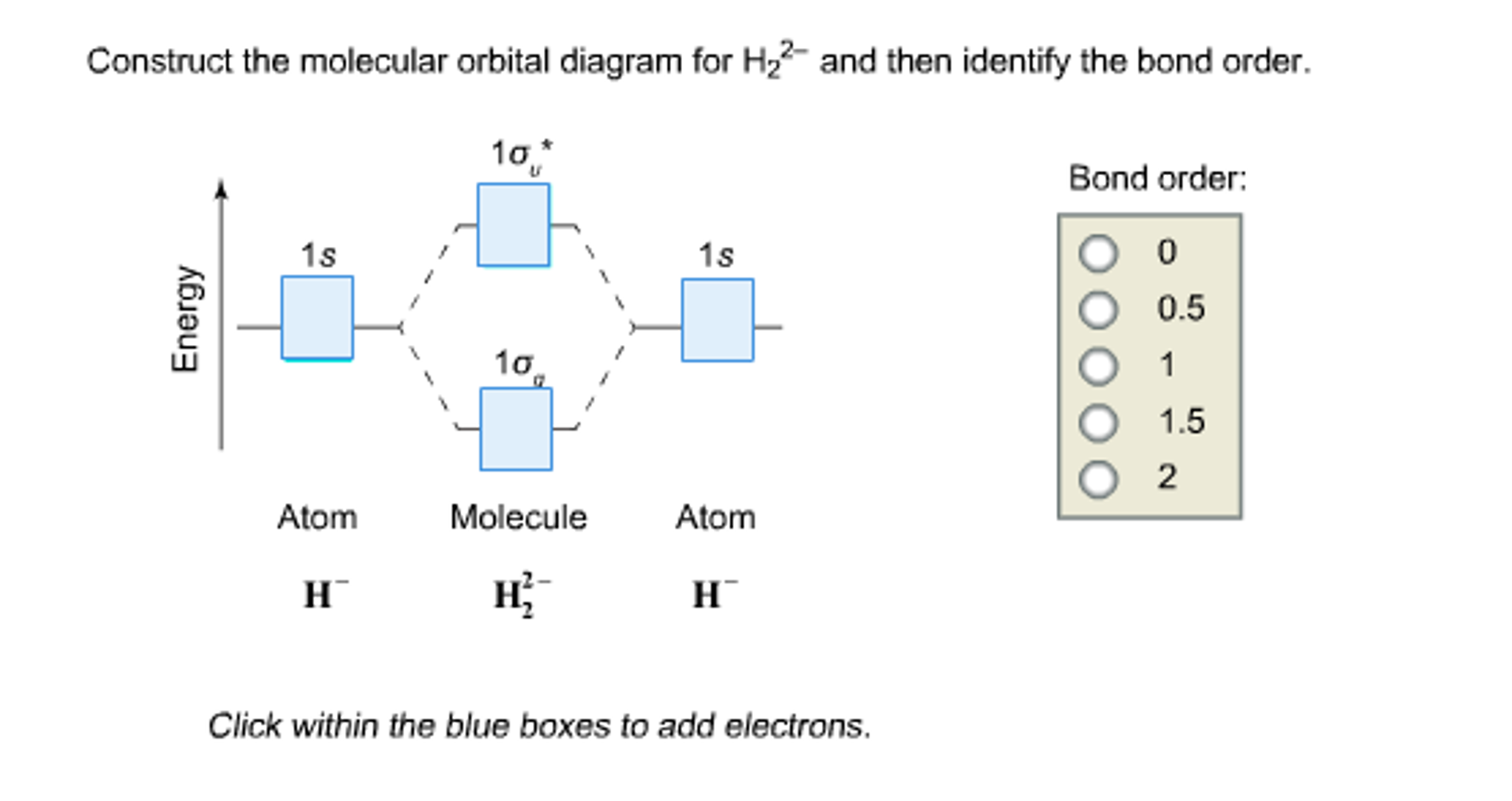
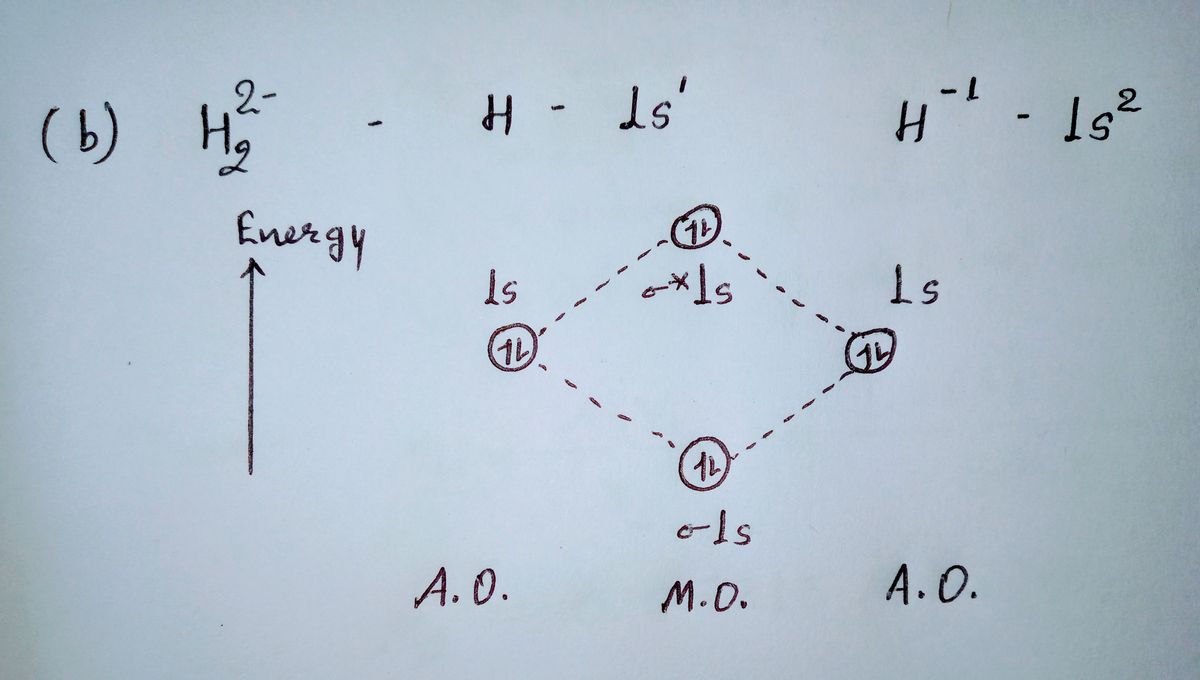
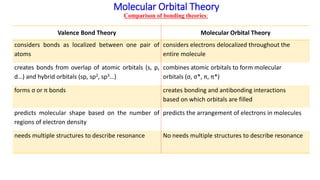
41 construct the molecular orbital diagram for h22– and then identify the bond order.
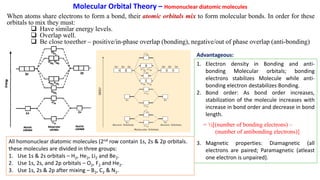
Answered: 4. Write a molecular orbital diagram,… | bartleby Solution for 4. Write a molecular orbital diagram, determine the bond order, and predict the magnetism for the followin C22- b. H22- а. c. Nez+ c. 022+ 43 construct the molecular orbital diagram for h2 below ... Chapter 2 - Molecular Orbital Theory - ppt video online download Use molecular orbital theory to predict molecular geometry for simple triatomic systems Rationalize molecular structure for several specific systems in terms of 35 Construct the MO diagram for FHF- Polyatomic molecules Recall: N atomic orbitals yields N molecular orbitals.
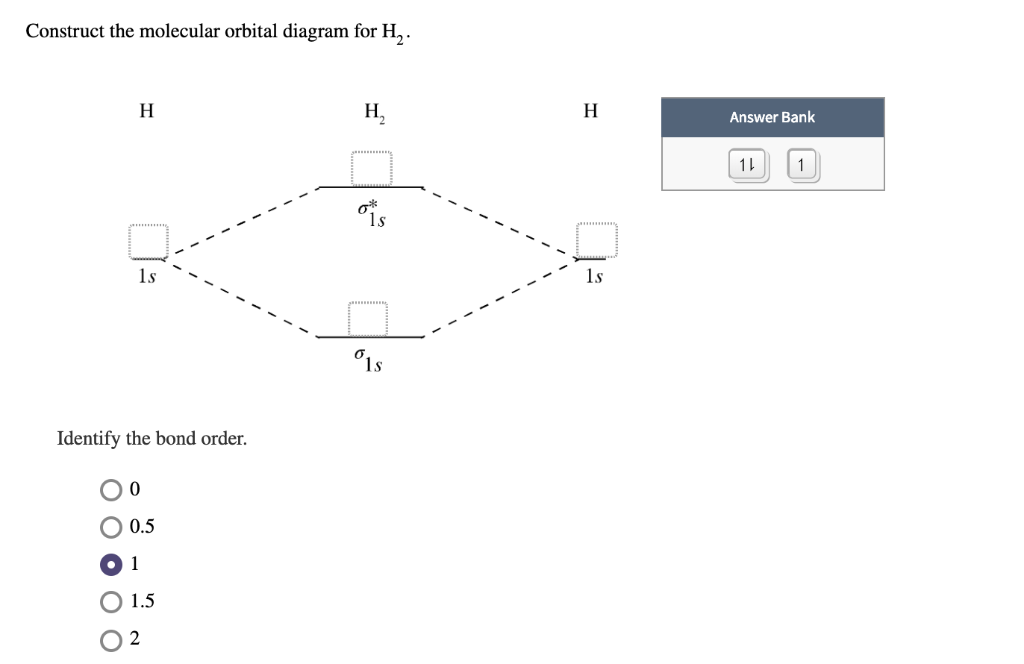
Construct the molecular orbital diagram for H22- and then identify the bond order. If you can't find your institution, please check your spelling and do not use abbreviations. If your institution is not listed, please visit our Digital Product Support Community .
Construct the molecular orbital diagram for h22– and then identify the bond order.
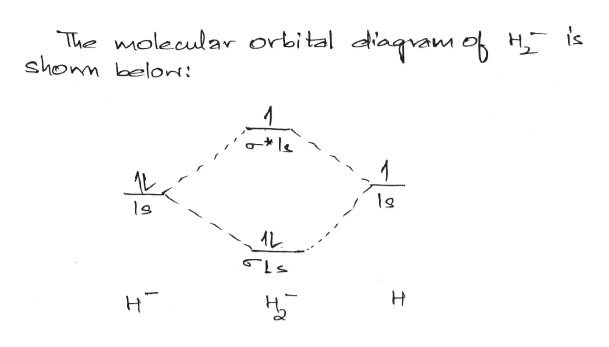
Solved Construct the molecular orbital diagram for H22 ... Construct the molecular orbital diagram for H22+ and then identify the bond order. Bond order: Energy OOOOO Atom Molecule Atom h* * * Click within the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H22+ and then identify the bond order. Bond order: Energy OOOOO Atom Molecule Atom h* * * Click within the blue ... electronic configuration - Molecular orbital (MO) diagram ... The short answer is: we could not tell it using the primitive molecular orbital theory introduced in the general chemistry courses. In exact same way we could not tell why $\mathrm{\sigma_{2p_{z}}}$ MO becomes lower in energy than $\mathrm{\sigma_{2p_{z}}}$ MO to the left of $\ce{N2}$ and not to the left of, say, $\ce{C2}$. How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
Construct the molecular orbital diagram for h22– and then identify the bond order.. He2 2+ Molecular Orbital Diagram - schematron.org 1 ½ e. 2 Construct the molecular orbital diagram for H 2- a What charge would be needed on F 2 to. Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Figure \(\PageIndex{1}\): Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1s Atomic Orbitals. Molecular Orbital Theory - Build H22- - YouTube For the ion H22-:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion Answered: Use molecular orbital theory to… | bartleby Identify this molecule and then determine the bond order. ... place the N and H3 orbitals on either side of a molecular orbital energy-level diagram. Then use your judgement about the effect of bonding and anti bonding interactions and energies of the parent orbitals to construct the molecular orbital energy levels in the centre of your diagram ... Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.
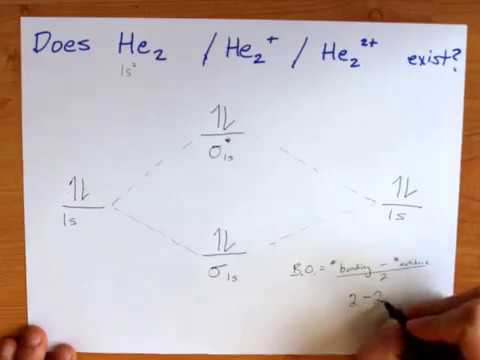
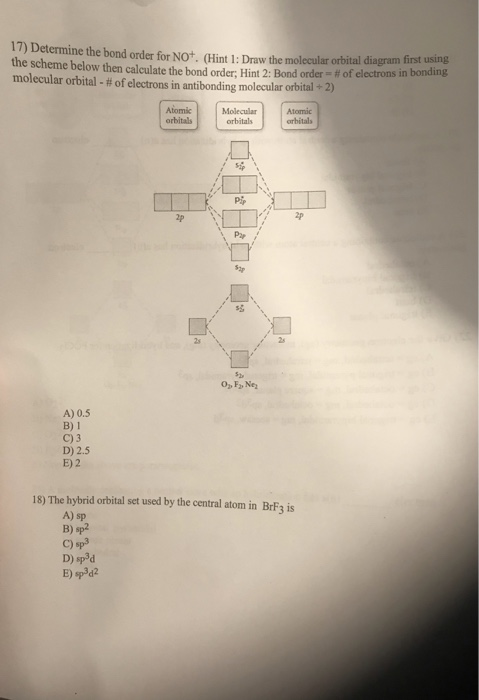
Best HW 8: Bonding Theories & IMFs Flashcards | Quizlet Which of the following statements concerning molecular orbital theory is true? 1. Bonding orbitals are lower in energy than their corresponding anti-bonding orbitals. 2. If a molecule has an odd number of electrons, then it is paramagenetic. 3. The MO diagrams for O2, F2, Ne2 are NOT filled using the Aufbau principle. Lecture 24: Molecular Orbital Theory I. Variational ... And in the next lecture, we're going to use molecular orbital diagrams like this to describe a series of examples. Homonuclear diatomic molecules, heteronuclear AH, and AB. H is special, and this is more general. And what we'd like to be able to do is without a great deal of thought construct the molecular orbital diagram for basically any ... chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2. Although there is a bonding influence from the two bonding electrons, there ... Bond order of c2+ ,c2 2- - Brainly.in Ace. 10.8K answers. 133.6M people helped. Bond order = 1/2 (number of electrons in bonding orbitals - number of electrons in antibonding orbitals) Therefore, Bond order of C2+ = 1/2 (5 - 2) = 3/2 = 1.5. Bond order of C2- = 1/2 (7 - 2) = 5/2 = 2.5. Bond order of C2 = 1/2 (6 - 2) = 2. Highest bond order means highest bond energy and shortest bond ...
H2S Lewis Structure, Molecular Geometry, Hybridization ... The repulsion changes the bond pairs from a straight to bent shape. All these explain the molecular geometry of H2S. H2S Molecular Orbital (MO) Diagram. The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. 41 molecular orbital diagram for h2 2- - Diagram For You Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. 38 molecular orbital diagram for h2 2- - Wiring Diagrams ... Solved Construct the molecular orbital diagram for... | Chegg.com Transcribed image text : Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: 0 0.5 1 1.5 2 Click within the blue boxes to add electrons. Mo Oxygen For Diagram [I3TUOB] Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. The more reactive a metal is towards oxygen, the more vigorously it burns in oxygen. Fill from the bottom up, with 12 electrons total. Check out the following example of O2 MO diagram below!.
Sapling Self Assessment 13 - Sapling Self Assessment 1 ... Construct the molecular orbital diagram for H22 and then identify the bond order. Click within the blue boxes to add electrons. 6. Construct the molecular orbital diagram for He2 and then identify the bond order. Click within the blue boxes to add electrons. 7.
8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Oxygen For Mo Diagram [6YLU2I] Search: Mo Diagram For Oxygen. About For Oxygen Diagram Mo
H2S Lewis Structure, Molecular Geometry, Hybridization and ... H2S Molecular geometry. Hybridization of the given molecule H2S is sp3; the Sulfur atom is in center bonding with two Hydrogen atoms forming the bond angle less than 180 degrees. According to the VSEPR theory, the lone pairs of electrons repel each other, but as the Sulfur atom is less electronegative, the bond angle decreases to 104.5 degrees.
Diagram Mo For Oxygen [Q0EUC6] In order to draw oxygen's molecular orbital diagram, you need to start by taking a look at what atomic orbitals you have for an oxygen atom, O. Carbon monoxide in terms of molecular orbital theory. The carbon atom goes on one side of the diagram while the oxygen SALCs are drawn on the opposite side. Molecular Orbital Diagram for Oxygen Gas (O2).
Solved Construct the molecular orbital diagram for H22 ... Construct the molecular orbital diagram for H 22+ and then identify the bond order. Thanks. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (8 ratings)
Molecular Orbital Diagrams simplified | by Megan A. Lim ... Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…
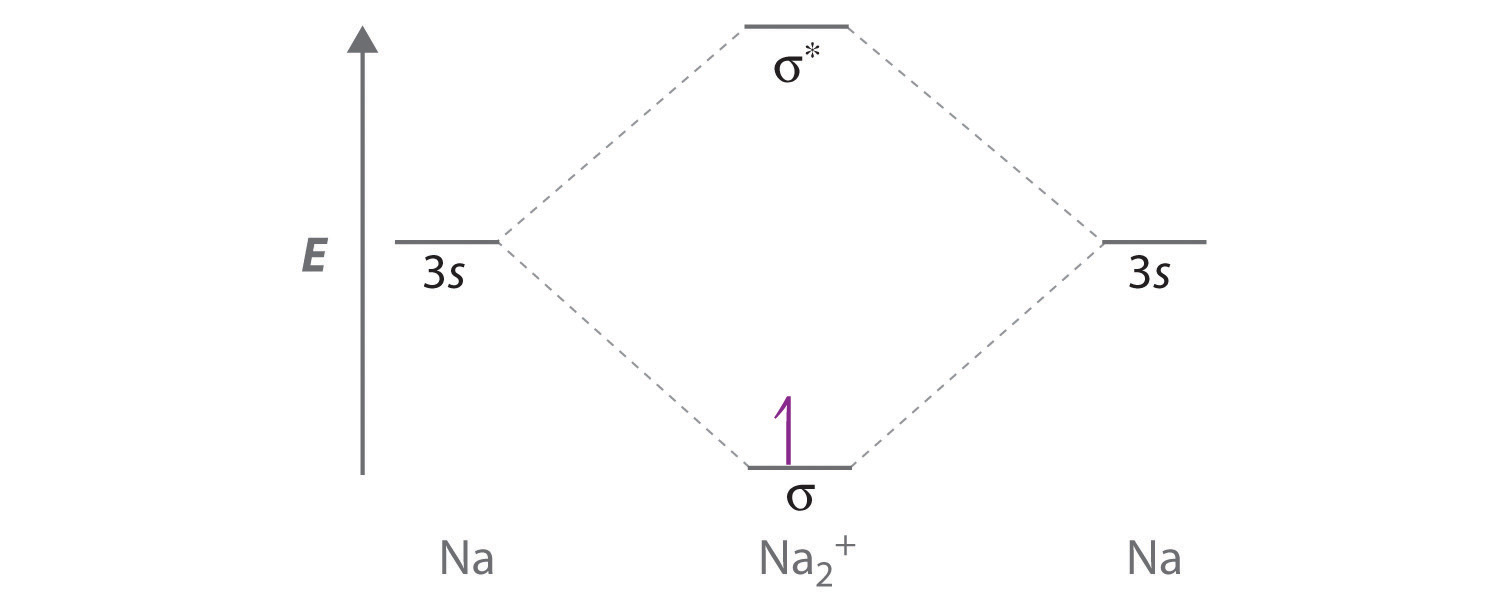
9.7: Molecular Orbitals - Chemistry LibreTexts Asked for: molecular orbital energy-level diagram, bond order, and stability. Strategy: Combine the two He valence atomic orbitals to produce bonding and antibonding molecular orbital; s. Draw the molecular orbital energy-level diagram for the system. Determine the total number of valence electrons in the He 2 2 + ion. Fill the molecular ...
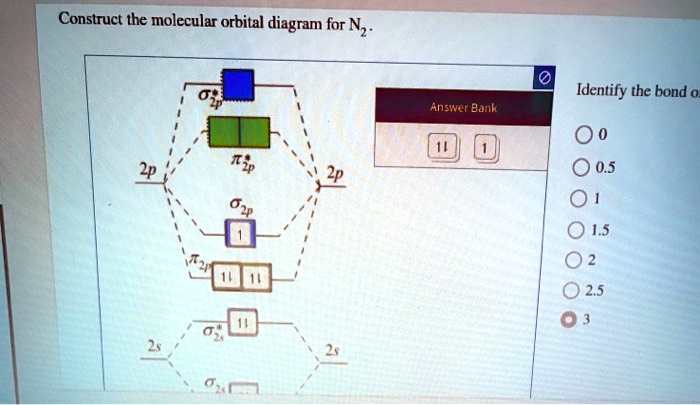
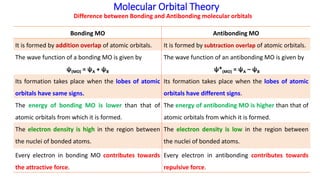
Energy level diagram for Molecular orbitals - Chemical ... 2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals. Bond Order = ½ ( N b - Na) The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero.
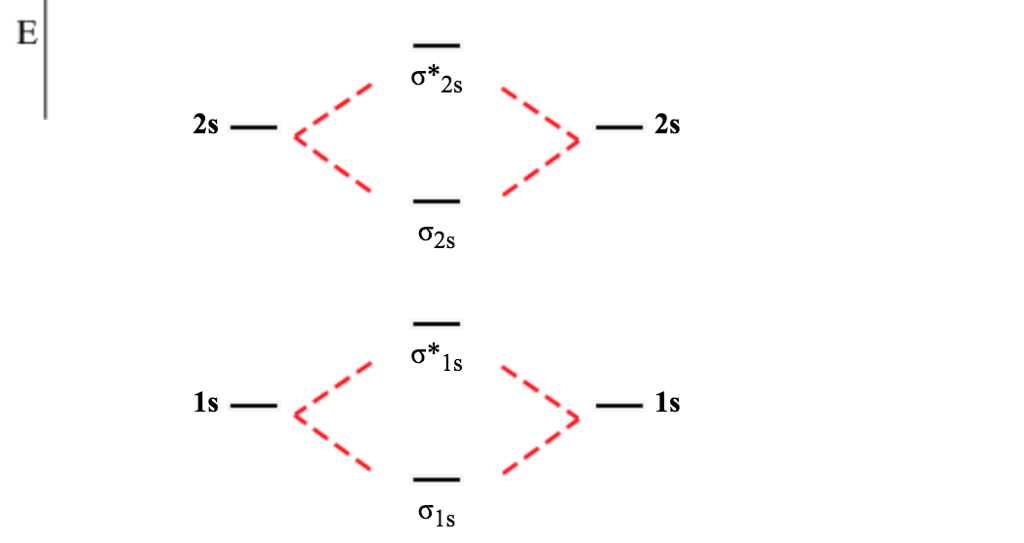
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.
How do I calculate the bond order for H2- and H2+? | Socratic Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
electronic configuration - Molecular orbital (MO) diagram ... The short answer is: we could not tell it using the primitive molecular orbital theory introduced in the general chemistry courses. In exact same way we could not tell why $\mathrm{\sigma_{2p_{z}}}$ MO becomes lower in energy than $\mathrm{\sigma_{2p_{z}}}$ MO to the left of $\ce{N2}$ and not to the left of, say, $\ce{C2}$.
Solved Construct the molecular orbital diagram for H22 ... Construct the molecular orbital diagram for H22+ and then identify the bond order. Bond order: Energy OOOOO Atom Molecule Atom h* * * Click within the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H22+ and then identify the bond order. Bond order: Energy OOOOO Atom Molecule Atom h* * * Click within the blue ...



















0 Response to "41 construct the molecular orbital diagram for h22– and then identify the bond order."
Post a Comment