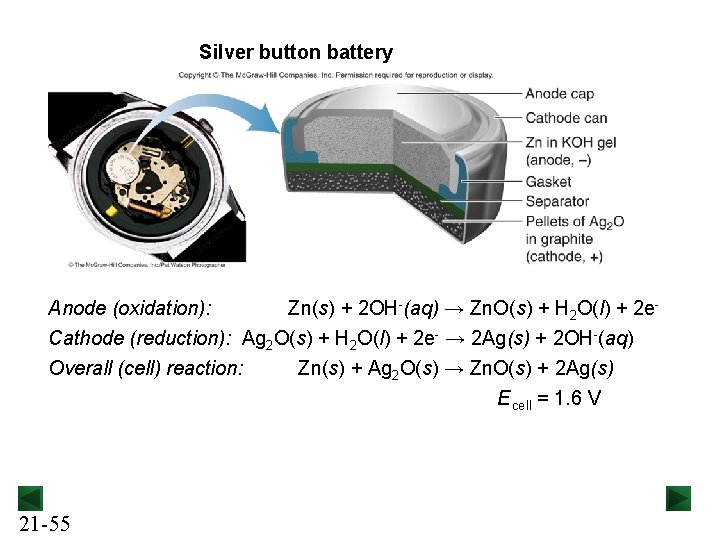
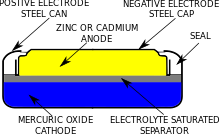
39 the cell diagram for the reaction occurring in silver-zinc button batteries is
Zinc-air battery - Wikipedia The reactions produce a theoretical 1.65 volts, but this is reduced to 1.35-1.4 V in available cells. Zinc-air batteries have some properties of fuel cells as well as batteries: the zinc is the fuel, the reaction rate can be controlled by varying the air flow, and oxidized zinc/electrolyte paste can be replaced with fresh paste. Electrical Cells and Battery - Electronics Tutorials These kinds of batteries have high energy density in the range of 100-120 WattHour / Kilo Gram. Batteries like Lead-acid batteries, nickle-iron batteries silver-zinc batteries etc falls in this kind and also various high-temperature batteries are under development which also falls under this kind of batteries. 3. Stationary Batteries.
Answered: For A arrows products, time and… | bartleby A: Interpretation - For the given cell diagram for the reaction occurring in silver-zinc button batter... question_answer Q: If 23.2 moles of oxygen react how many liters of diphosphorous pentaoxide can form at STP?

The cell diagram for the reaction occurring in silver-zinc button batteries is
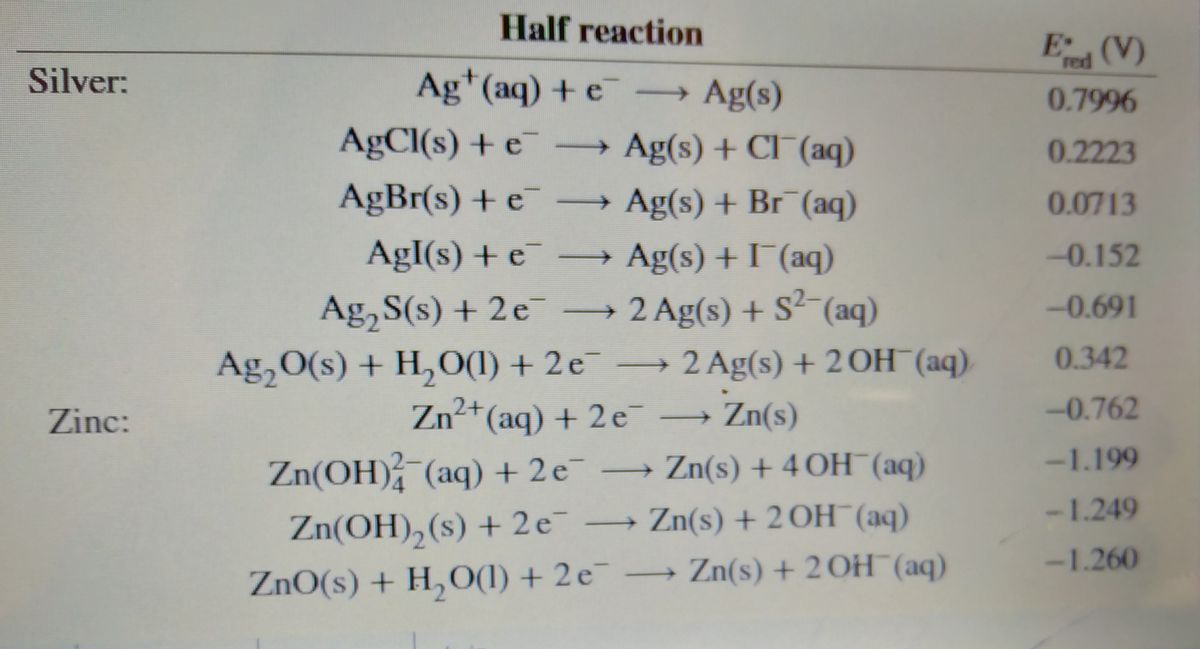
The cell diagram for the reaction occurring in silver-zinc ... 1) Calculate the cell potential for the following reaction as written at 25.00 °C, given that [Cr2 ] = 0.897 M and [Sn2 ] = 0.0190 M. Standard reduction potentials can be found here. ( ) Cr (s) + Sn2+ (aq) <=> Cr2+ (aq) + Sn (s) 2. Calculate the cell potential for the following reaction ... The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOH(aq)|Ag2O(s)|Ag(s) (a) Write the half-reaction equation that involves silver. (b) Write the half-reaction equation which involves zinc. (c) Write the balanced equation for the net cell reaction equation. (d) Calculate the value of E°cell. Which one of the following statements concerning the ... This cycle consists of the following steps: Analyze and journalize transactions. Post the journal entries to the general ledger accounts. Prepare a trial balance. Journalize and post the adjusting entries. Prepare an adjusted trial balance. Prepare financial statements. Journalize and post the closing entries. Prepare a post-closing trial balance.
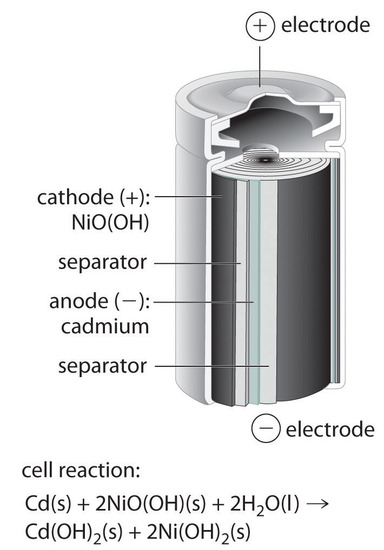
The cell diagram for the reaction occurring in silver-zinc button batteries is. PDF Batteries - American Institute of Physics The cell half reactions are MH(s) + OH-(aq) → M(s) + H 2 O(l) + e (oxidation) NiOOH(s) + H 2 O(l) + e-→ -Ni(OH) 2 (s) + OH (aq) (reduction) and the overall cell reaction is M(s) + 2 Ni(OH) 2(s) discharge recharge MH(s) + NiOOH(s) Figure U.2 Metallic dendrites formed during electrochemical deposition. The cell diagram for the reaction occurring in silver zinc ... The cell diagram for the reaction occurring in silver zinc button batteries is {eq}Zn(s)|ZnO(s)|KOH(aq)|Ag_2O(s)|Ag(s){/eq} a) Write the half-reaction that involves silver. DOCX Student Name: - VCE CHEMISTRY The equation for the reaction occurring in zinc, silver oxide button cells is. Zn(s) + Ag. 2 O(s) + H 2 O(l) 2Ag(s) + Zn(OH) 2 (s)Question 2. In this cell. ... The diagram below comes from a description of a new cutting edge material. It is a polymer solid but it allows conduction as ions can move through it. ... Write balanced half-equations ... The cell diagram for the reaction occurring in silver-zinc ... Mar 25, 2022 · Chem question. The cell diagram for the lead-acid cell that… Definition of Electrochemistry - What it is, Meaning and… In the chemical equation Zn + 2HCl ZnCl + H, the reactants… Definition of Stack - What is, Meaning and Concept; A _____ accelerates a chemical reaction in a cell. substrate… Definition of Anode - What is, Meaning and ...
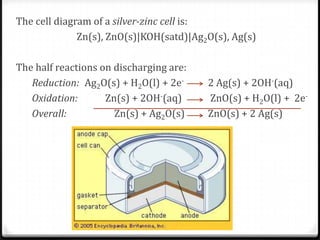
1 The Leclanché Dry Cell The electrolyte is a moist paste ... 39 (3) The Silver Zinc Cell: A Button Battery The cell diagram of a silver zinc cell (Fig. 19-16) is The half-reactions on discharging are 40 (4) The Nickel Cadmium Cell: A Rechargeable Battery • The nickel cadmium cell (or nicad battery) is commonly used in cordless electric devices, such as electric shavers and handheld calculators. Solved The cell diagram for the reaction occurring in | Chegg.com The cell diagram for the reaction occurring in silver-zinc button batteries is Zn (s)|ZnO (s)|KOH (aq)|Ag2O (s)|Ag (s) (a) Write the half-reaction equation that involves silver. (b) Write the half-reaction equation which involves zinc. (c) Write the balanced equation for the net cell reaction equation. Daniell Cell - Definition, Construction & Working with ... Here current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. Daniell cell is a reversible cell while a voltaic cell may be reversible or irreversible. Cell Reactions. Ion Zn/ZnSO 4 half cell, oxidation reaction occurs. Zn → Zn 2+ + 2e - Ion Cu/CuSO4 half cell, reduction reaction occurs. Cu 2 ... Question : Question The CCl4 formed in the ... - ScholarOn Question The cell constant of a conductivity sell is equal L/A. The resistance of the NaCl solution is found to be 254 Ohm. Estimate the NaCl... Question The cell diagram for the reaction occurring in silver-zinc button batteries is shown below (a) Write the half-reaction equation that involves silver (b) write the...
Solved The cell diagram for the reaction occurring in | Chegg.com The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOHW|Ag2O(S)|Ag(S) Write the half-reaction equation that involves silver. Write the half-reaction equation which involves zinc. Write the balanced equation for the net cell reaction equation. (Get Answer) - Question 2 Figure Q2 shows the Pourbaix ... The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOH(aq)|Ag2O(s)|Ag(s) (a) Write the half-reaction equation that involves silver. (b) Write the half-reaction equation which involves zinc. (c) Write the... Galvanic Cell Diagrams Chemistry Tutorial - AUS-e-TUTE By convention, the cell diagram is written with the anode on the left hand side. Oxidation (loss of electrons) occurs at the anode . Mg(s) loses 2 electrons to form Mg2+ at the anode. Reduction (gain of electrons) occurs at the cathode . Ag+ gains 1 electron to form Ag(s) at the cathode. Write the equation for the reduction of Ag+ The cell diagram for the reaction occurring in silver-zinc ... 1) Calculate the cell potential for the following reaction as written at 25.00 °C, given that [Cr2 ] = 0.897 M and [Sn2 ] = 0.0190 M. Standard reduction potentials can be found here. ( ) Cr (s) + Sn2+ (aq) <=> Cr2+ (aq) + Sn (s) 2.
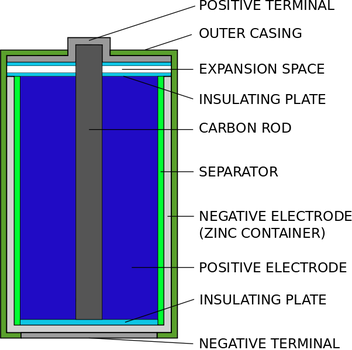
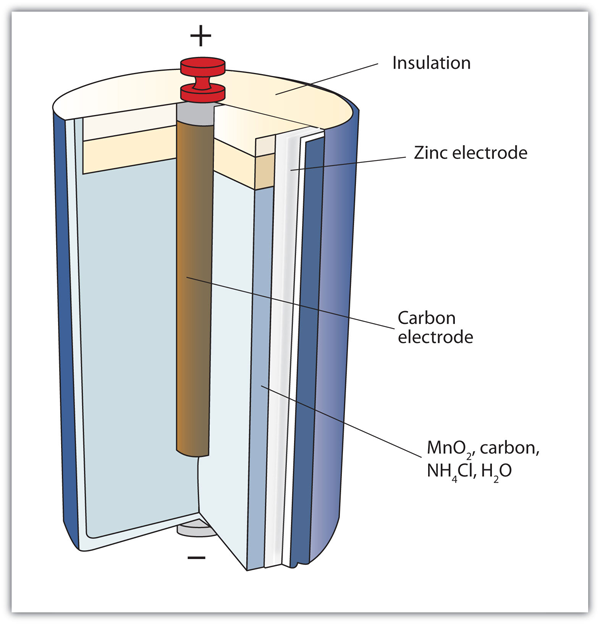
Zinc-carbon battery - Wikipedia A zinc-carbon battery (or carbon zinc battery in U.S. English) is a dry cell primary battery that provides direct electric current from the electrochemical reaction between zinc and manganese dioxide (MnO 2) in the presence of an electrolyte. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a ...
The cell diagram for the reaction occurring in silver-zinc ... The cell diagram for a silver-zinc button battery is Zn(s), ZnO(s) |KOH(aq) Ag, O(s), Ag(s) Write the balanced half-reaction that occurs at the anode during discharge. anode half-reaction: Write the balanced half-reaction that occurs at the cathode...
OneClass: The cell diagram for the reaction occurring in ... Get the detailed answer: The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOH(aq)|Ag2O(s)|Ag(s) a) Write the hal
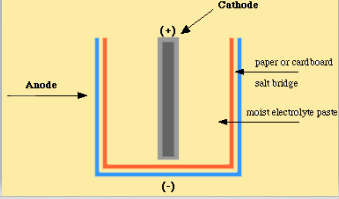
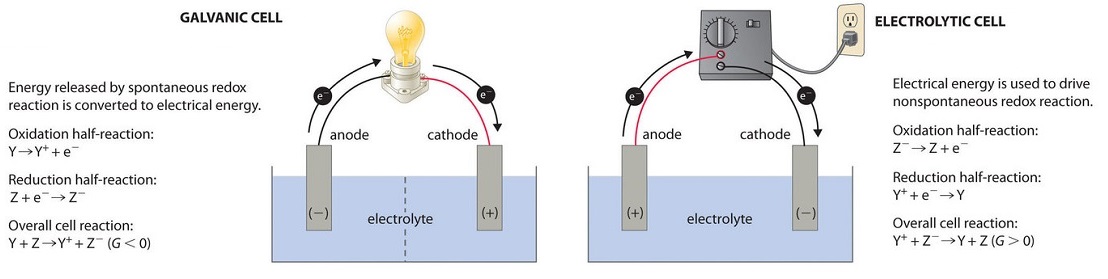
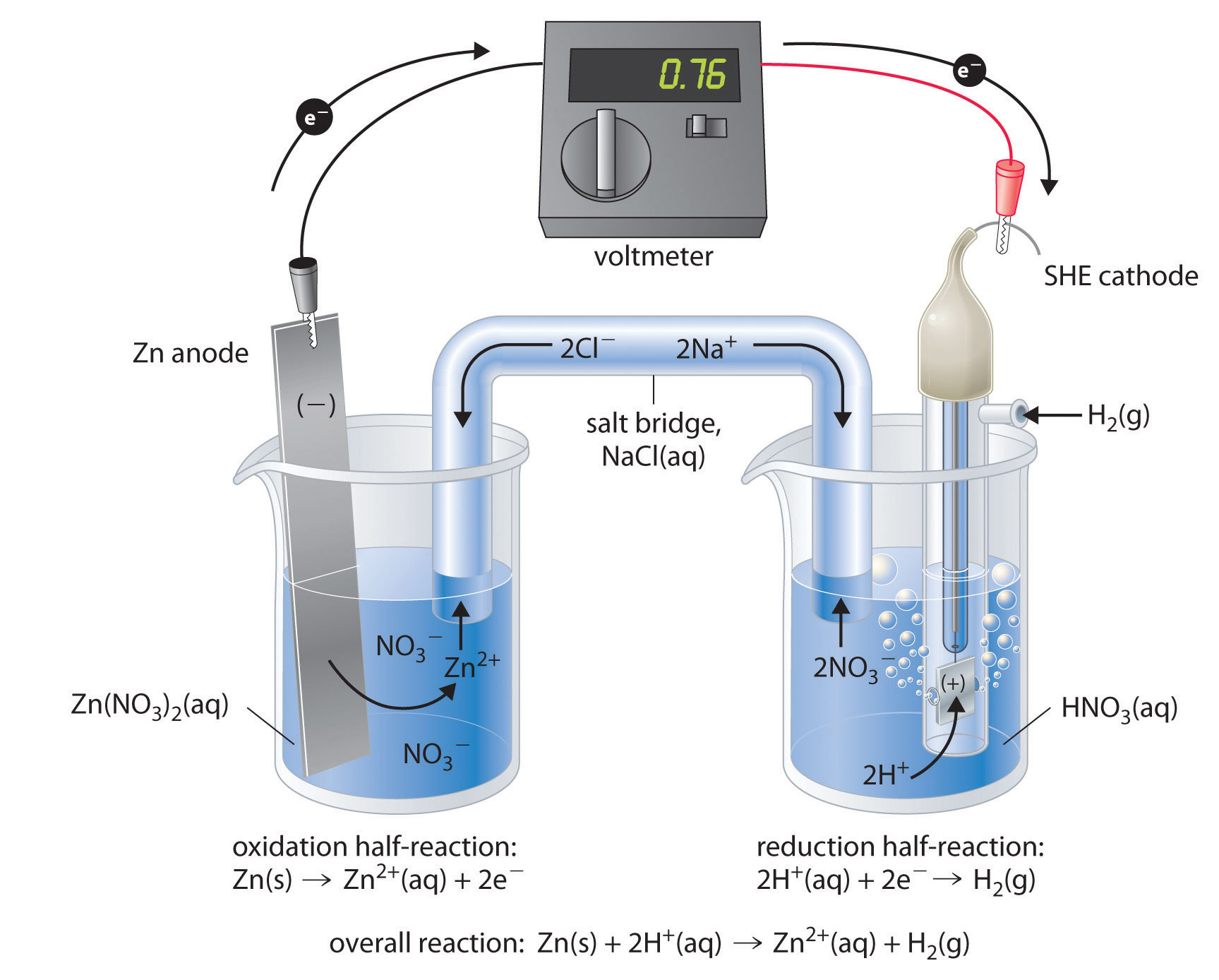
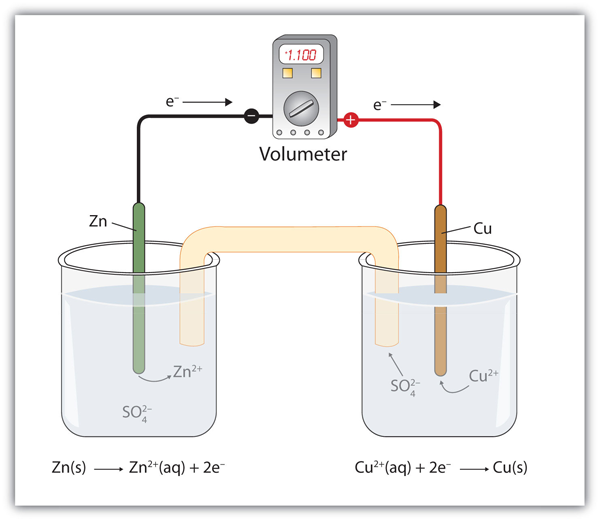
Batteries Chemistry Tutorial - AUS-e-TUTE The cell is a galvanic cell ( spontaneous redox reaction occurs). Chemical energy is converted to electrical energy. Oxidation occurs at the anode. Anode is negative ( − ). Reduction occurs at the cathode. Cathode is positive ( + ). Electrons flow from anode (-) to cathode (+).
Answered: Fill in the product, starting material,… | bartleby A: Interpretation - For the given cell diagram for the reaction occurring in silver-zinc button batter... question_answer Q: Calculate the pH of a solution that is 0.66 M HF and 1.00 M KF.
Definition, working principle and types of dry cell - BYJUS The overall reaction is of the cell. Zn + HgO → ZnO + Hg. 4. Silver oxide cell. In the basic medium, silver metal acts as inert support in the reduction of silver oxide (Ag 2 O) and in the oxidation of zinc. Step 1: Reaction at the cathode. Ag 2 O + 2H + + 2e - → 2Ag + H 2 O. Step 2: Reaction in the electrolyte. 2H 2 O → 2H + + 2OH ...
Where did the phrase "inquiring minds want to know" come ... They would intersperse headlines of the day with the voice-over guy saying the phrase and actors proclaiming, "*I* want to know.". I am not absolutely positive that is the origin, but it's the first place I ever heard it, in the mid-70's. "Inquiring minds want to know" originally came from E. F. Hutton (Stock Brokers) commercials on TV.
Batteries | Boundless Chemistry - Lumen Learning A common dry-cell battery is the zinc-carbon battery, which uses a cell that is sometimes called the Leclanché cell. The cell is made up of an outer zinc container, which acts as the anode. The cathode is a central carbon rod, surrounded by a mixture of carbon and manganese(IV) dioxide (MnO 2). The electrolyte is a paste of ammonium chloride ...
Coolerman Wiring Fj40 Wiring Diagram - Complete basic car included (engine bay, interior and exterior lights, under dash harness, starter and ignition circuits, instrumentation, etc). Jan 04, · The diagram shows an optional seperate flasher for the haz and inline fuse between the haz and the stop fuse. scanned all the diagrams for Coolerman..
Silver Zinc Batteries - an overview | ScienceDirect Topics The positive silver electrode is made from sintered silver powder or silver oxide converted into metallic silver. Its cycle life depends on the depth of discharge and temperature, and it can exceed 5000 cycles under favorable conditions. The overall electrochemical cell reaction is AgO + Zn + H 2 O → Zn ( OH) 2 + Ag
The Cell Diagram For The Reaction Occurring In Silver-zinc ... The cell diagram for the reaction occurring in silver-zinc button batteries is Question 1) Calculate the cell potential for the following reaction as written at °C, given that [Cr2 ] = M and [Sn2 ] = M. Standard reduction potentials can be found here.
Which one of the following statements concerning the ... This cycle consists of the following steps: Analyze and journalize transactions. Post the journal entries to the general ledger accounts. Prepare a trial balance. Journalize and post the adjusting entries. Prepare an adjusted trial balance. Prepare financial statements. Journalize and post the closing entries. Prepare a post-closing trial balance.
Calculate the cell potential for the following reaction ... The cell diagram for the reaction occurring in silver-zinc button batteries is Zn(s)|ZnO(s)|KOH(aq)|Ag2O(s)|Ag(s) (a) Write the half-reaction equation that involves silver. (b) Write the half-reaction equation which involves zinc. (c) Write the balanced equation for the net cell reaction equation. (d) Calculate the value of E°cell.
The cell diagram for the reaction occurring in silver-zinc ... 1) Calculate the cell potential for the following reaction as written at 25.00 °C, given that [Cr2 ] = 0.897 M and [Sn2 ] = 0.0190 M. Standard reduction potentials can be found here. ( ) Cr (s) + Sn2+ (aq) <=> Cr2+ (aq) + Sn (s) 2.





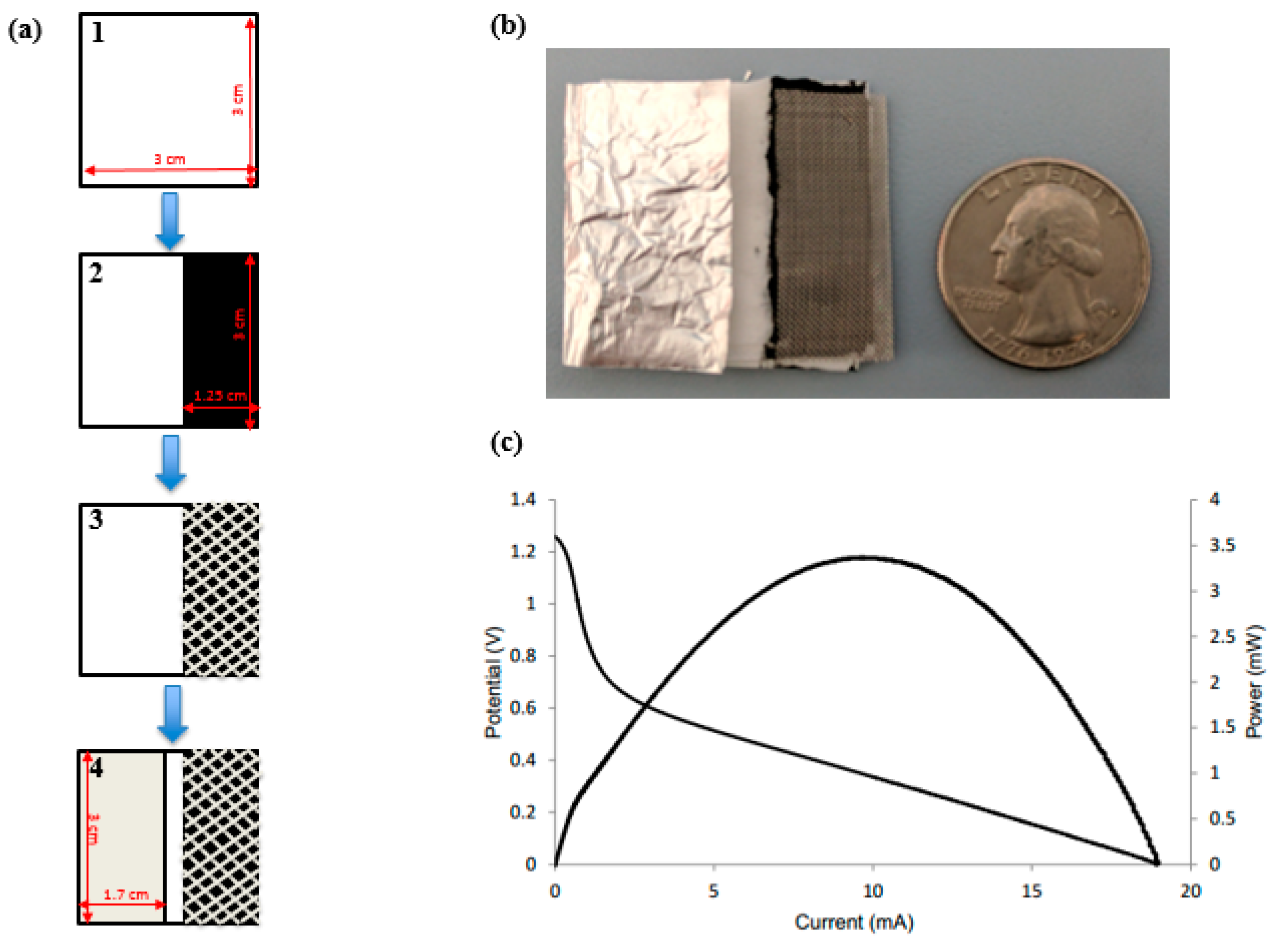
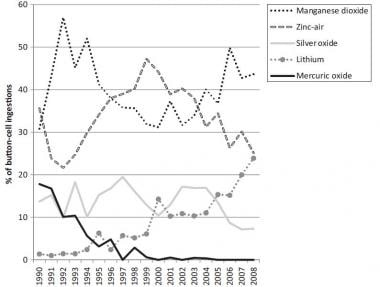

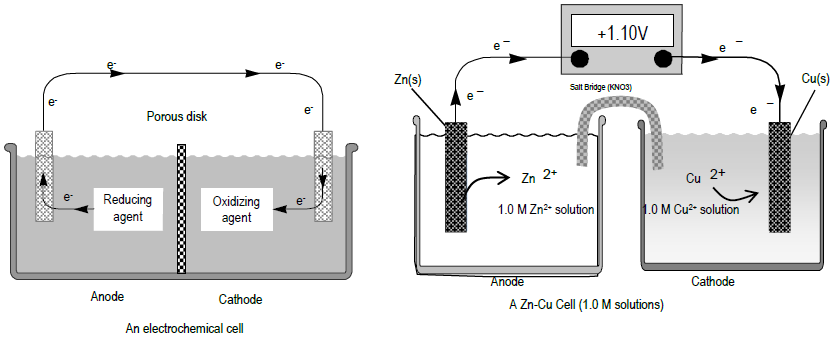





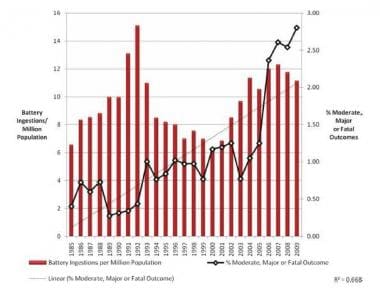



0 Response to "39 the cell diagram for the reaction occurring in silver-zinc button batteries is"
Post a Comment