42 silicon electron dot diagram
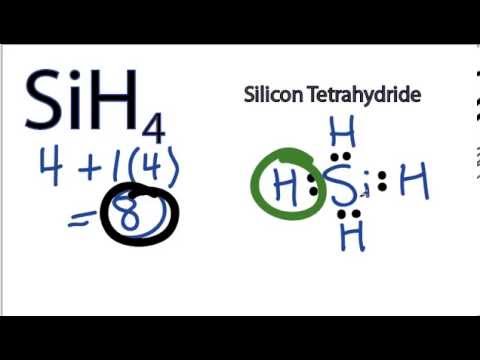
Lewis Electron Dot Diagrams - GitHub Pages (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. What is the Lewis dot structure for silicon? How is it ... Answer (1 of 2): Silicon is in Group 14 (sometimes called Group IV or 4). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon you'll put four "dots" or valance electrons around the element symbol (Si).
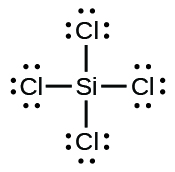
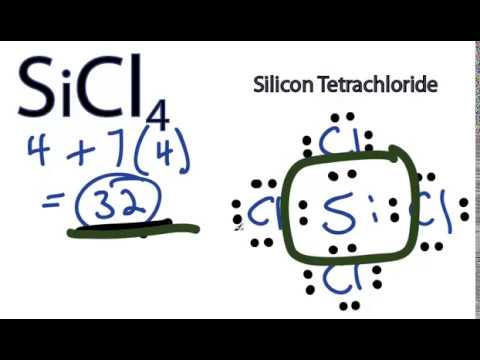
SiCl4 Lewis structure, Molecular geometry, Bond angle ... There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. Let's see how to draw this step by step- Follow some steps for drawing the lewis dot structure of SiCl4 1. Count total valence electron in SiCl4
Silicon electron dot diagram
Draw the Lewis structure of silicon tetrafluoride. What ... Lewis Structure: A Lewis structure is a diagram that describes the covalent bonding between two or more atoms present in a molecule or ion. The atoms are written in terms of their elemental symbols. What is the electron dot diagram for silicon? - Answers silicon What is the electron dot structure for silicon? There are four valance electrons in Silicon, therefore there will be 4 dots in your electron dot diagram. In an electron dot diagram of... Silicon: Element Lewis Structure, Facts & Discovery ... Silicon is the 14th element on the periodic table Silicon is a metalloid or semi-metal, which is a group of metals that have some properties of metals and some properties of nonmetals. Pure silicon...
Silicon electron dot diagram. Periodic Table of Elements: Silicon - Si ... Atomic Structure of Silicon. Atomic Radius: 1.46 Å. Atomic Volume: 12.1cm 3 / mol. Covalent Radius: 1.11 Å. Cross Section (Thermal Neutron Capture) σ a / barns: 0.171. Crystal Structure: Cubic face centered. Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 2. The Lewis structure for silicon disulfide is to be ... The number of valence electron present in silicon ( Si) atom is four. The number of valence electron present in sulfur ( S) is six. The number of valence electron present in silicon disulfide ( SiS 2) is calculated as shown below. No. of valence electron in SiS 2 = Si + 2S = 4 e − + 2 × 6 e − = 16 e − SiCl4 Lewis Structure - How to Draw the Lewis Structure ... A step-by-step explanation of how to draw the SiCl4 Lewis Dot Structure (Silicon tetrachloride).For the SiCl4 structure use the periodic table to find the to... Electron Configuration for Silicon (Si) - UMD In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Lewis Structures ... 100+ Lewis Structures Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons. SiO2 Lewis Structure, Molecular Geometry, Hybridization ... Silicon's electronic configuration = 1s2 2s2 2p6 3s2 3p2 Hence, No. of valence electrons in oxygen = 6 No. of valence electrons in Silicon = 4 The total number of valence electrons available in the Lewis structure of SiO2 is 4 + 2*6 = 16 electrons. Step 2: Find the atom with the least electronegativity and position it in the middle. Silicon carbide | SiC - PubChem Silicon carbide | SiC or CSi | CID 9863 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ... SiO2 Lewis Structure| Step By Step Construction - What's ... SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2.The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure.. Step-1: Count the valence electrons of atoms For the SiO 2 Lewis structure, we need to figure out the number of valence ...
Hee Sun drew an electron dot diagram of a silicon atom as ... These electrons dots are placed around the chemical symbol of that element. In electron dot structure of silicon, there are four dots present around the symbol 'Si' because their are four valence electrons present in the valence shell of the silicon atom. SiF4 Lewis Structure - Learnool SiF4 Lewis Structure. March 10, 2022 March 9, 2022 by Admin. SiF 4 (silicon tetrafluoride) has one silicon atom and four fluorine atoms. In the lewis structure of SiF 4, there are four single bonds around the silicon atom, with four fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... A. Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
SiS2 Lewis Structure, Molecular Geometry, Hybridization ... The atomic number of Silicon is 14, and the electronic configuration is 1s2 2s2 2p6 3s2 3p2. As p shell can accommodate a maximum of 6 valence electrons, there is a dearth of 4 electrons because of which the total number of valence electrons in silicon is 4.
Which model represents the electron dot diagram of silicon? L 1.4.2 Quiz: Scientific Practices Question 1 of 10 In a controlled experiment, which group experiences the test? O A. Measurements and observations O …. B. Experimental group O c. Control group O D. Instruments and equipment. sodium chloride has a boiling point of what degree celcius. 18) Identify compound (C) in the following synthetic ...
Lewis Dot Symbols and Lewis Structures | Boundless Chemistry Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
What is the electron dot diagram for aluminum ... What is electron shell diagram? For that, we have electron shell diagrams. For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.
SiO2 Lewis Structure - Learnool In the periodic table, silicon lies in group 14, and oxygen lies in group 16. Hence, silicon has four valence electrons and oxygen has six valence electrons. Since SiO 2 has one silicon atom and two oxygen atoms, so… Valence electrons of one silicon atom = 4 × 1 = 4 Valence electrons of two oxygen atoms = 6 × 2 = 12
How to make a Lewis structure out of SiC when both (Si and ... Answer (1 of 7): Silicon carbide (rarely: the mineral moissanite) is a refractory solid with a number of different allotropic covalent network structures. All of them have the atoms bound to four neighbors in a tetrahedral fashion with four covalent \sigma-bonds to the neighboring atom. One struc...
Silicon Bohr Model - How to draw Bohr diagram for Silicon ... Electron dot diagram of a Silicon atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Silicon, we got to know, it has only 4 valence electrons. So, just represent the 4 valence electrons around the Silicon atom as a dot. The electron configuration of Silicon
Lewis Dot Structure for Silicon Atom (Si) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for Si (Silicon). I show you where Silicon is on the periodic table and how to determine h...
Silicon tetraiodide | SiI4 - PubChem Silicon tetraiodide | SiI4 or I4Si | CID 83498 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ...
What is the electron dot structure for silicon? - Answers silicon In an electron dot diagram of potassium There is one dot. In an electron dot diagram of silicon there are four dots. Which element would you expect to be more reactive? silicon Synonym for...
Silicon: Element Lewis Structure, Facts & Discovery ... Silicon is the 14th element on the periodic table Silicon is a metalloid or semi-metal, which is a group of metals that have some properties of metals and some properties of nonmetals. Pure silicon...
What is the electron dot diagram for silicon? - Answers silicon What is the electron dot structure for silicon? There are four valance electrons in Silicon, therefore there will be 4 dots in your electron dot diagram. In an electron dot diagram of...
Draw the Lewis structure of silicon tetrafluoride. What ... Lewis Structure: A Lewis structure is a diagram that describes the covalent bonding between two or more atoms present in a molecule or ion. The atoms are written in terms of their elemental symbols.








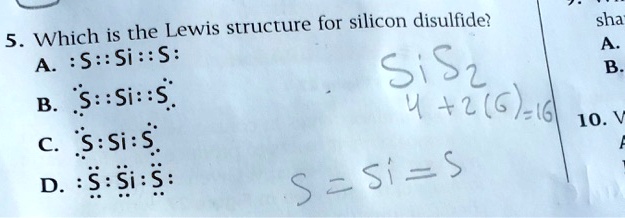




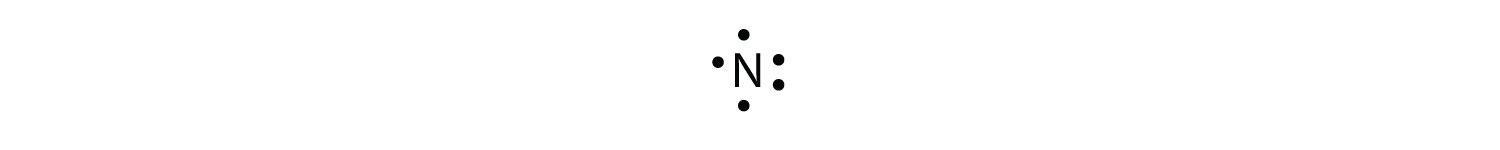

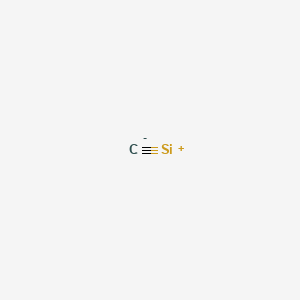


![Silicon carbide]](https://www.degruyter.com/document/doi/00.0000/IUPAC.iupac.compound.9863/asset/images/9863.png)
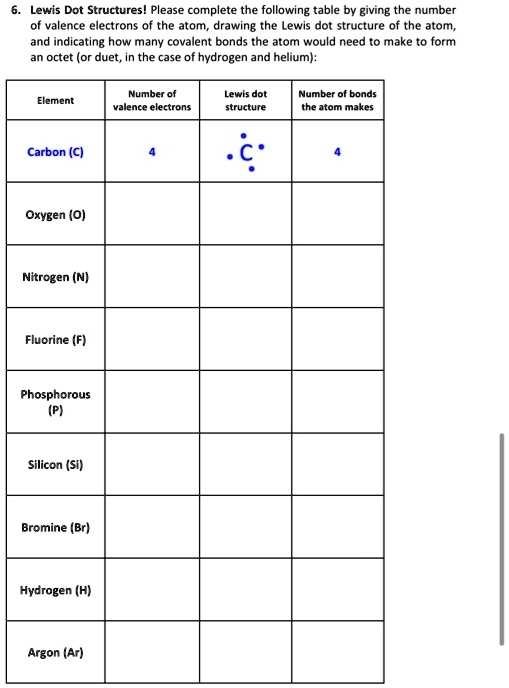
0 Response to "42 silicon electron dot diagram"
Post a Comment