42 lewis dot diagram for n2
techiescientist.com › nh3-lewis-structureNH3 Lewis Structure, Geometry, and Hybridization ... Mar 05, 2022 · The lewis structure that is also called an electron dot structure, is mainly a pictorial representation of the valence electrons present in an atom. The diagram is drawn using dots around the symbol of an atom, mostly in pairs. Solved How to draw the Lewis structure for N2+ | Chegg.com Question: How to draw the Lewis structure for N2+ This problem has been solved! See the answer See the answer See the answer done loading. How to draw the Lewis structure for N2+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality ...
Answered: Draw the Lewis Dot Structure for N2.… | bartleby Homework help starts here! Browse 5+ million homework and textbook solutions, concept explainers, videos and more. Search concepts or drop in your homework problem! Our library grows every minute-keep searching! Science Chemistry Q&A Library Draw the Lewis Dot Structure for N2. What type of bond keeps this molecule together (single, double, or ...
Lewis dot diagram for n2
Draw the Lewis dot structures of N2 and CCl4 - Brainly.in 1) Lewis dot structure of N2 is given below: (a) In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. (b) So N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. 2) Lewis dot structure of CCl4 is given below: What is the Lewis Structure for N2 (nitrogen gas)? - Quora Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron... Draw electron dot structure of CO2,H2O,F2,N2 Draw electron dot structure of CO 2 ,H 2 O,F 2 ,N 2 . Draw electron dot structure of. C.
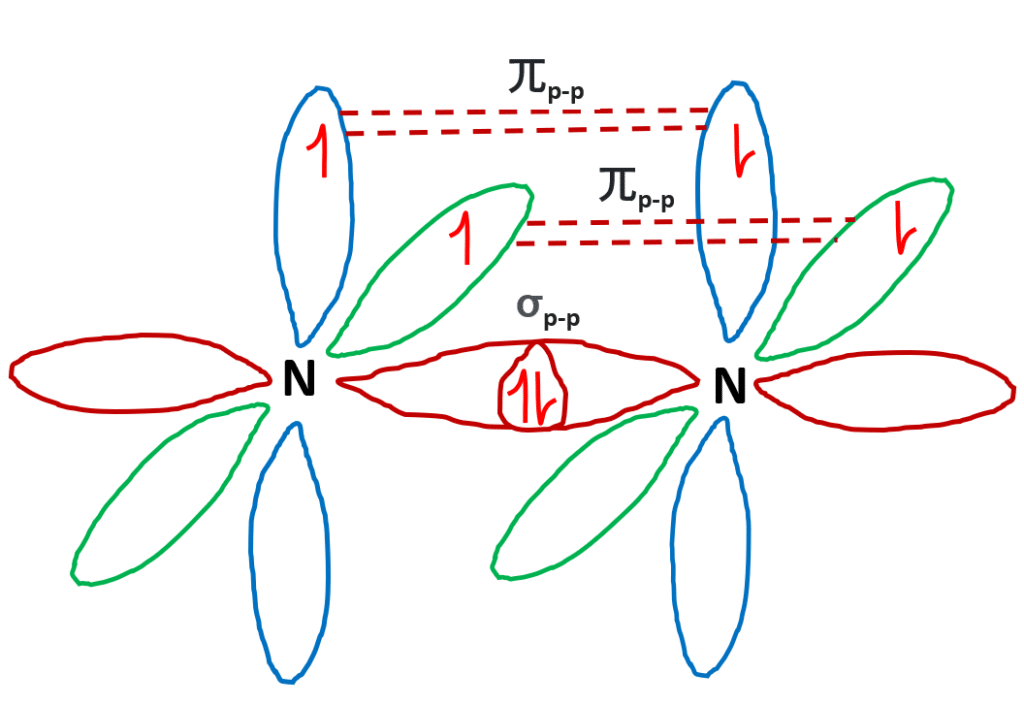
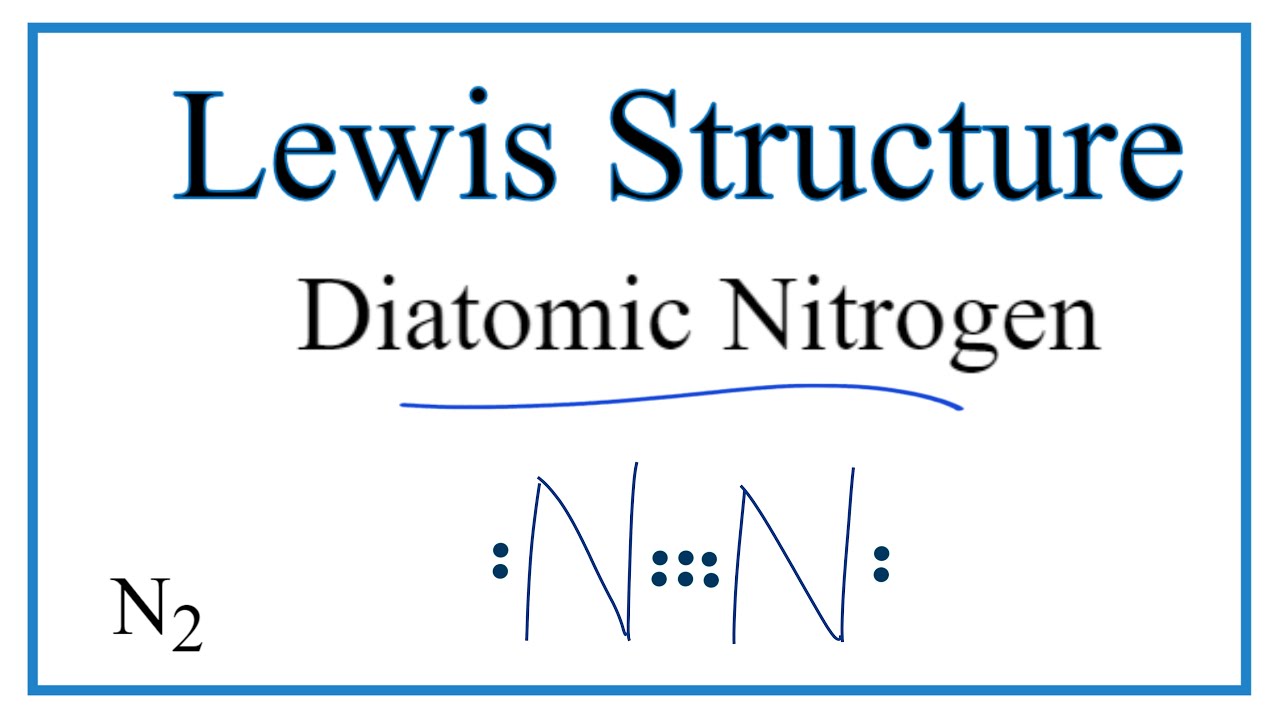
Lewis dot diagram for n2. What is the Lewis structure for N2? - Answers What is the Lewis dot formation of N2? The N2 molecule consists of two nitrogen atoms held together by a triple bond. Each nitrogen atom also has a lone-pair of electrons. N2 Lewis Structure: Full Guide (2022 Updated) N2 Lewis Structure: What You Need To Know. The Lewis structure of the N2 would consist of two Nitrogen atoms connected by a triple bond. The octet rule states that nitrogen atoms must link three times. The N2 molecule is diatomic, Each Nitrogen atom has one lone pair of electrons. Nitrogen has five valence electrons in the N2 electron dot ... Is N2 polar or nonpolar: Nitrogen polarity explained ... The Lewis dot structure for Nitrogen will be this: However, when two atoms of Nitrogen bind together, it has the following structure: Here both these atoms share six valence electrons to form a triple bond. These electrons are shared equally, and both Nitrogen atoms have one lone pair of electrons. N2 Polarity topblogtenz.com › cyanide-cn-lewis-structureCN- lewis structure, molecular orbital diagram, and, bond order In this article, we will study the Cyanide (CN-) lewis structure, molecular orbital diagram(MO), its bond order, formal charges, and hybridization. Cyanide can be a colorless gas in the form of hydrogen cyanide, sodium cyanide, potassium cyanide, etc.
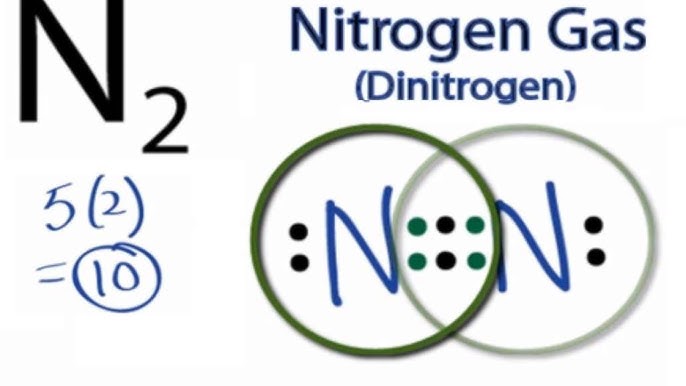
N2 Lewis Structure | Lewis Structure N2 | HND Assignment The following is the Lewis Dot Structure for N2, 2N and N2, are basically two forms of the same element. There is a little difference between the two. 2N refers to two molecules of Nitrogen atom, and N2 states that two atoms of Nitrogen are present in a single molecule. The number written at the start, refers to the number of molecules and the ... techiescientist.com › n2-lewis-structureN2 Lewis Structure, Molecular Geometry, and Hybridization ... 2 days ago · Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen. What is the Lewis structure of N2? | Socratic In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-. ai-team.it › keccyai-team.it Feb 23, 2022 · N2 lone pairs
PDF Lewis dot structure of n2 molecule Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2. N2 Lewis Structure: How to Draw the Dot Structure for N2 ... Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond. Lewis Dot Diagram For N2 - schematron.org on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . quizlet.com › 556589717 › mid-term-chem-flash-cardsMid Term Chem Flashcards | Quizlet Lewis electron-dot diagrams for CO2 and SO2 are given above. The molecular geometry and polarity of the two substances are A. the same because the molecular formulas are similar B. the same because C and S have similar electronegativity values C. different because the lone pair of electrons on the S atom make it the negative end of a dipole
Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
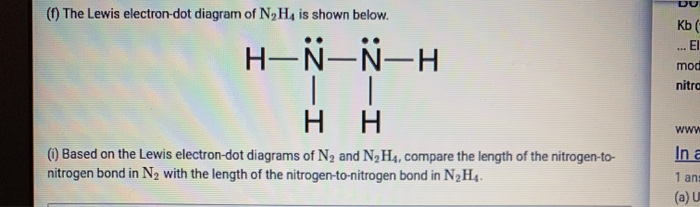
dink-magazin.de › drawing-ionic-bonds-worksheetdink-magazin.de 2 days ago · Quiz 5. Worksheets Lewis Dot Structures For each of the following, draw the Lewis Dot Structure, give the electron arrangement (E. N2o5 dinitrogen pentoxide 11. A Lewis Dot Structure Ionic Bonds Worksheet is a few short questionnaires on an individual topic.
Lewis Structure of N2 (Nitrogen Gas) - YouTube How to Draw the Lewis Structure of N2 - with explanation!Check me out:
42 lewis dot diagram for n2 - Modern Wiring Diagram - Quora Following is the Lewis dot cross structure for Nitrogen (N2) In N2 Lewis structure,two nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone To draw the N2 Lewis structure , we have to find out the valence electrons of nitrogen first.We express valence electrons as...
study.com › learn › lewis-structure-questions-andLewis Structure Questions and Answers | Study.com Draw the Lewis dot structure for PF5 and provide the following information. a. number of atoms bonded to the central atom b. number of lone electron pairs on the central atom c. hybridization of th...
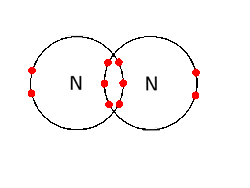
What is the Lewis dot structure for nitrogen gas ... What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ...
How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
Draw the Lewis structure for N2. Nitrogen is an unreactive ... The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair.
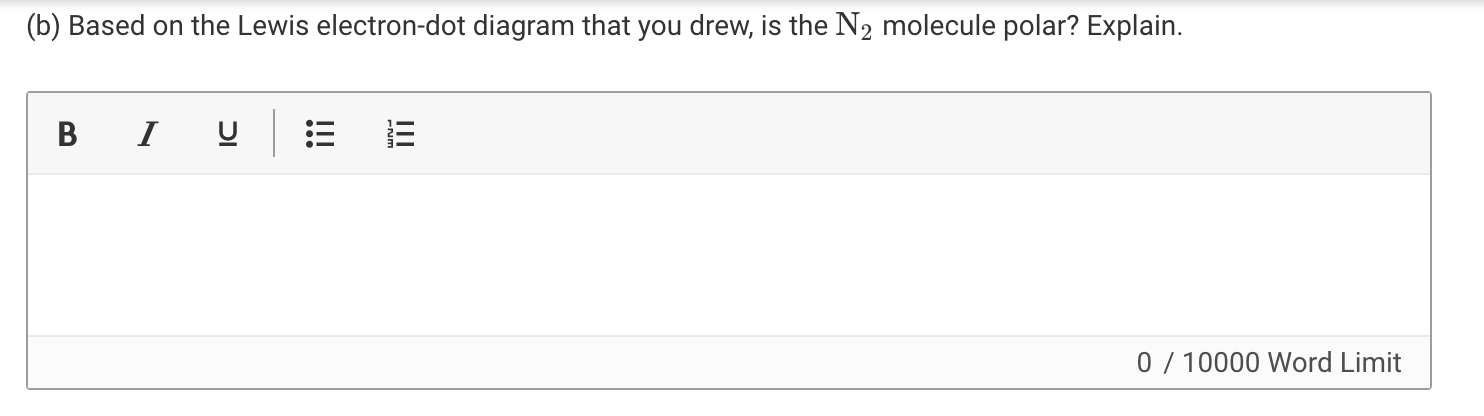
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Answered: Draw the Lewis Dot structure for N2 (on… | bartleby Science Chemistry Q&A Library Draw the Lewis Dot structure for N2 (on paper, not on Canvas) then answer the questions. a. How many total valence electrons are in N2? Express your answer as a whole number. b. How many single bonds are in N2? Express your answer as a whole number.
Nitrogen (N2) Molecule Lewis Structure Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
N2 Lewis Structure - draw the electron dot structure of o2 ... N2 Lewis Structure. Here are a number of highest rated N2 Lewis Structure pictures on internet. We identified it from reliable source. Its submitted by organization in the best field. We agree to this kind of N2 Lewis Structure graphic could possibly be the most trending subject past we part it in google improvement or facebook.
PPT PowerPoint Presentation - Chemical BONDING Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell!
N2 Lewis Structure - Easy Hard Science N2 Leweis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It's easiest to think in terms of...
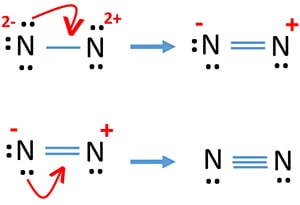
What is the Lewis dot structure for N2? - Answers However, the carbonate anion, CO3^2- does have a Lewis dot structure. What is the Lewis dot structure for N2 look like? N(triple bond)N and then two dots on each N in which ever spot is open.
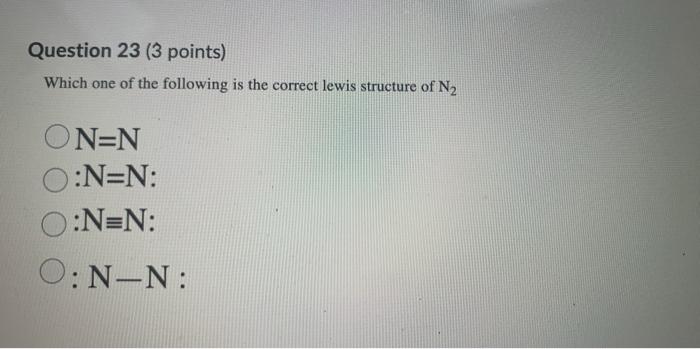
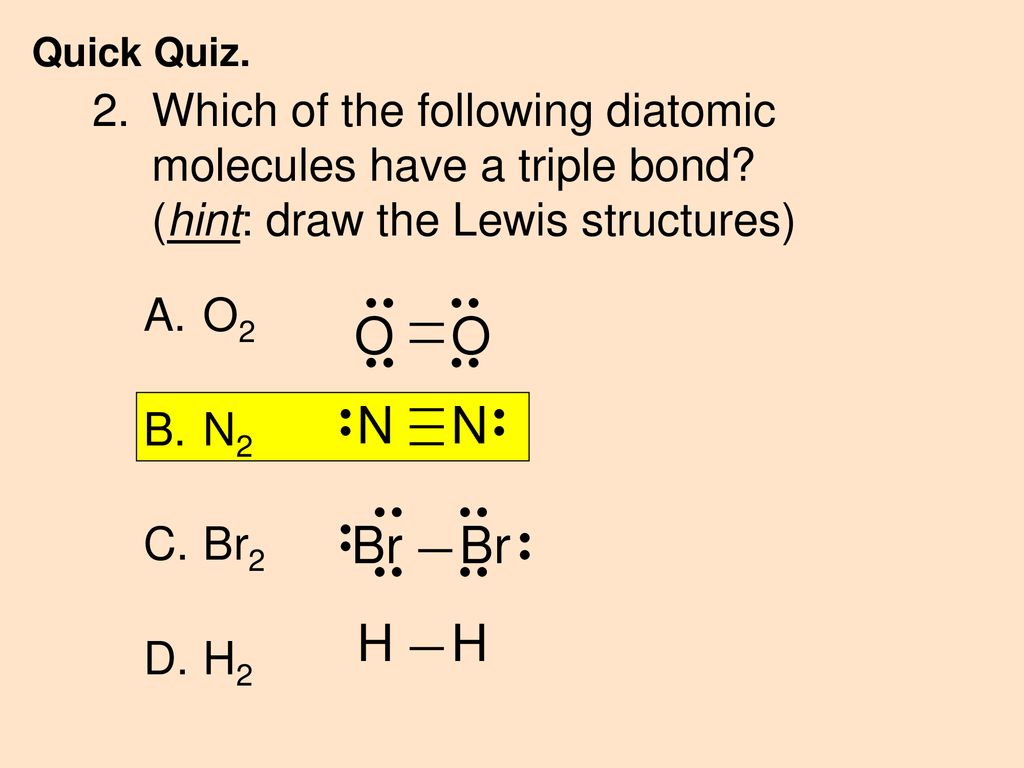
Solved In the Lewis electron dot structure for N2, what is ... In the Lewis electron dot structure for N2, what is the total number of additional dots (electrons) shown around each N atom? 2 Question 35 (1 point) Saved In the Lewis electron dot structure for Cl2, hqw many lines (representing bonds) should be shown between the two Cl atoms? 1 A/ Question 36 (1 point) Saved In the Lewis electron dot ...
Draw electron dot structure of CO2,H2O,F2,N2 Draw electron dot structure of CO 2 ,H 2 O,F 2 ,N 2 . Draw electron dot structure of. C.
What is the Lewis Structure for N2 (nitrogen gas)? - Quora Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron...
Draw the Lewis dot structures of N2 and CCl4 - Brainly.in 1) Lewis dot structure of N2 is given below: (a) In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. (b) So N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. 2) Lewis dot structure of CCl4 is given below:

![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1648865_1784763_ans_65460fbc5eda4a3b8021b75bbc2803a5.png)








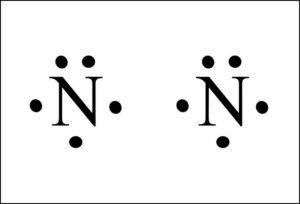









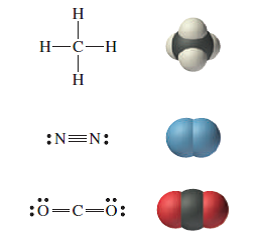



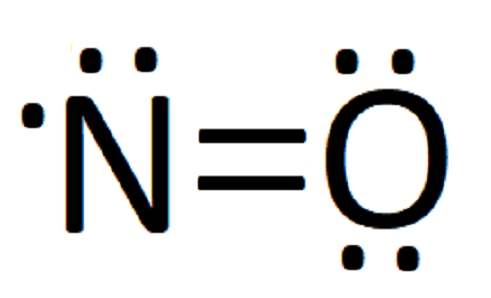
0 Response to "42 lewis dot diagram for n2"
Post a Comment