41 lewis electron dot diagram definition
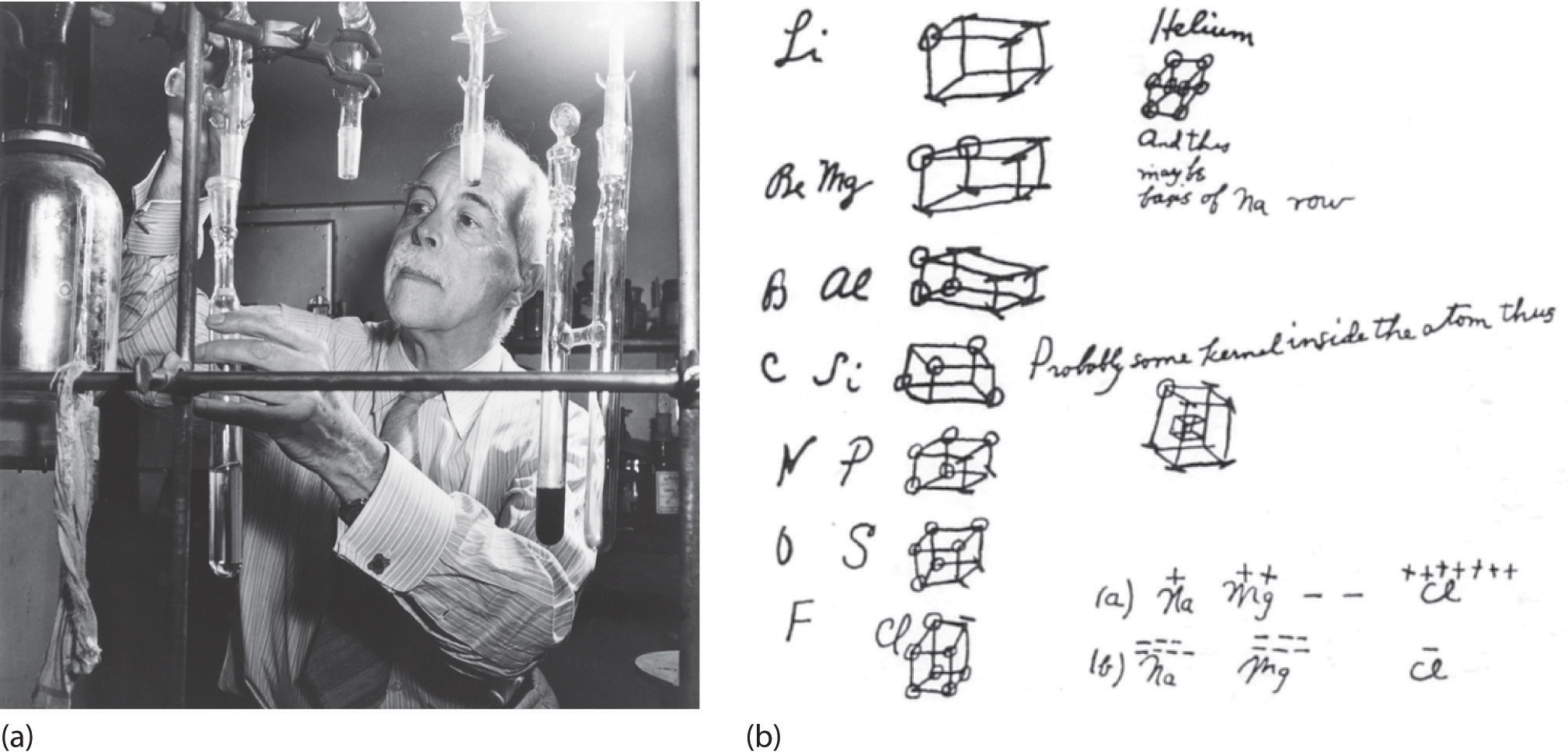
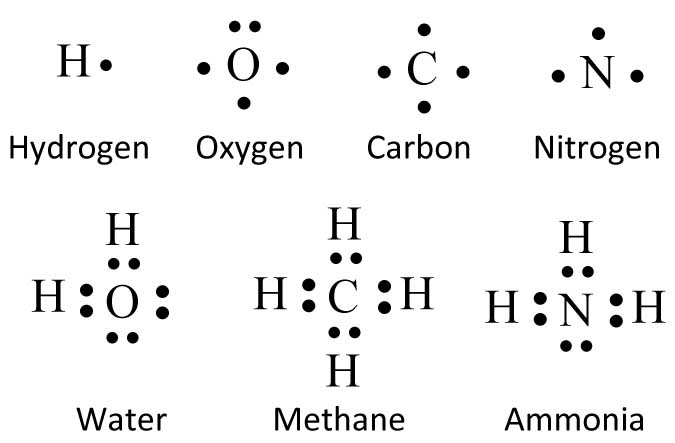
Lewis Electron-Dot Structures | Chemistry for Non-Majors In a previous chapter, you learned that the valence electrons of an atom can be shown in a simple way with an electron dot diagram. A hydrogen atom is shown as H• because of its one valence electron. The structures of molecules that are held together by covalent bonds can be diagrammed by Lewis ... Lewis Structures or Electron Dot Structures Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs.
Lewis Dot Structures: Definition, Structure and Sample ... Lewis dot structures or electron dot structures describe the chemical bonding amongst atoms inside a molecule with help of diagrams. Each of the atoms inside a molecule has lone pairs inside them, with the help of the lewis dot structure their total number can be displayed. Lewis dot structures are also known as electron dot or Lewis structures.

Lewis electron dot diagram definition
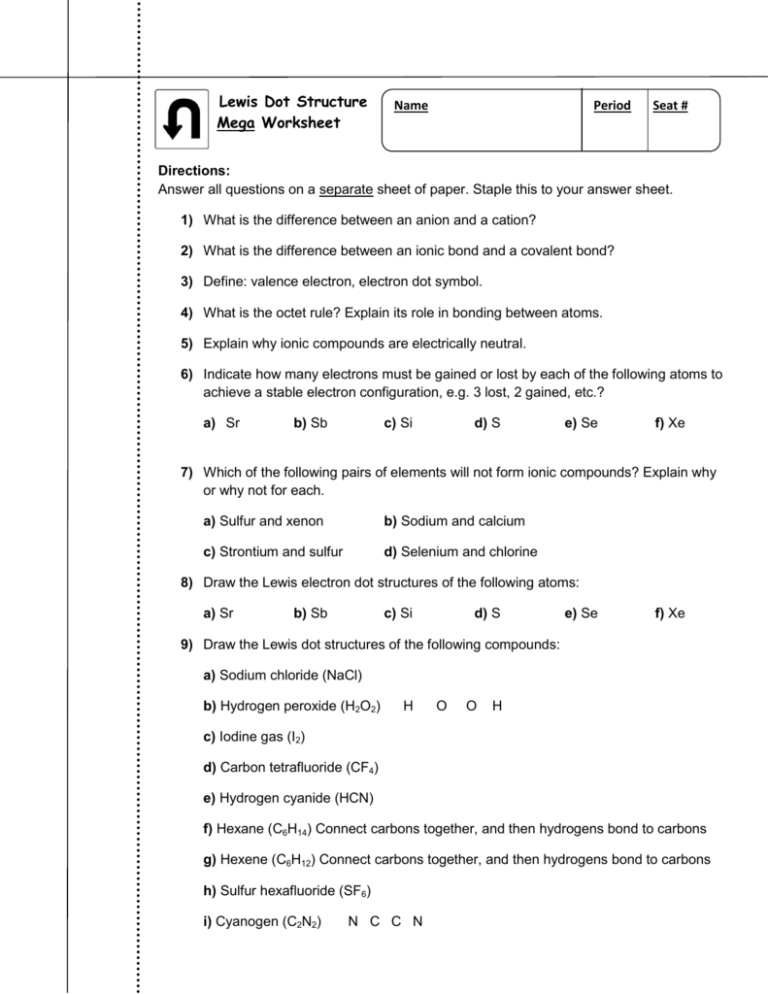
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Tetrahedral in Molecular Geometry: Definition, Structure ... Oct 22, 2021 · Use the following Lewis diagram for ethyl amine to answer the questions: Remember that geometry refers to the geometry defined by the atoms, not the electron pairs. The geometry about atom 1 is ... Definition of Lewis formula (electron dot or Lewis structure) A double bond is represented by two pairs of dots, etc. Dots representing non-bonded outer-shell electrons are placed adjacent to the atoms with which they are associated, but not between the atoms. Formal charges (e.g. +, -, 2+, etc.) are attached to atoms to indicate the difference between ...
Lewis electron dot diagram definition. Lewis Electron Dot Diagram - Concept - Chemistry Video by Brightstorm Time-saving video demonstration on how to draw Lewis Electron Dot Diagrams. Lewis Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. electron dot diagram in a sentence - electron dot diagram ... electron dot diagram in a sentence - Use electron dot diagram in a sentence and its meaning 1. The valence electrons can be counted using a Lewis electron dot diagram as shown at the right for carbon dioxide. 2. Lewis introduced the " electron dot diagrams " in this paper to symbolize the electronic structures of atoms and molecules. click for more sentences of electron dot diagram... Lewis structure (electron dot structure; dot structure ... Definition of Lewis structure (electron dot structure; dot structure) Search Web Search Dictionary Get Babylon's Dictionary & Translation Software Free Download Now! Lewis Dot Structure Flashcards | Quizlet In a Lewis Structure, a shared pair of electrons is depicted with a _____, an unpaired electron is depicted with a _____ by itself, and a lone pair of electrons is depicted with a _____ next to each other.
Valence Electrons and Lewis Electron Dot of Atoms and Ions Atomic Structure Links · Valence Electrons and Lewis Electron Dots of Atoms and Ions Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Double Covalent Bond | Facts, Definition, History & Examples Sep 27, 2019 · Lewis introduced the lewis dot structure, or Lewis notation or electron dot notation, in which the valence electrons i.e. those present in an outer shell was represented by dots around the atomic symbol and the pairs of electrons that were located between the atoms were represented by the covalent bonds. Lewis structure in chemistry 1 week ago - Lewis structures, also called electron-dot structures or electron-dot diagrams, are diagrams that show the bonding between atoms of a molecule, and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently-bonded molecule, as well as coordination ...
3.1: Lewis Electron-Dot Diagrams - Chemistry LibreTexts October 4, 2021 - The bonding between atoms in a molecule can be topically modeled though Lewis electron dot diagrams. Creating Lewis diagrams is rather simple and requires only a few steps and some accounting of the … Lewis Structures - Chemistry LibreTexts August 15, 2020 - A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons … Lewis Electron Dot Formulas 2 - Covalent Bonds - MCAT Content A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom's nucleus and are found in principal energy levels that contain only up to a specific number of electrons. Lewis structure - Wikipedia Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Lewis Structure Definition & Rules - What's Insight Lewis structure definition. A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between atoms. They are also known as are also called Lewis dot diagrams, electron dot diagrams, Lewis dot formulas, or electron dot formulas.
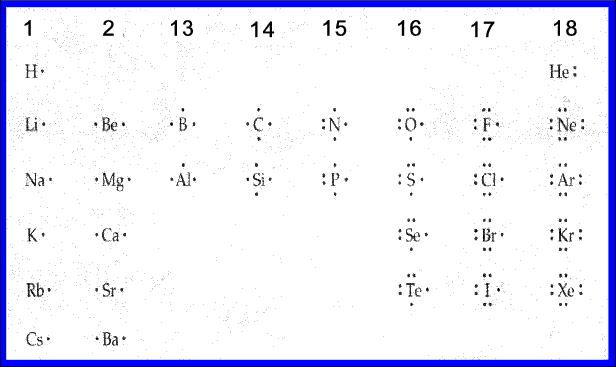
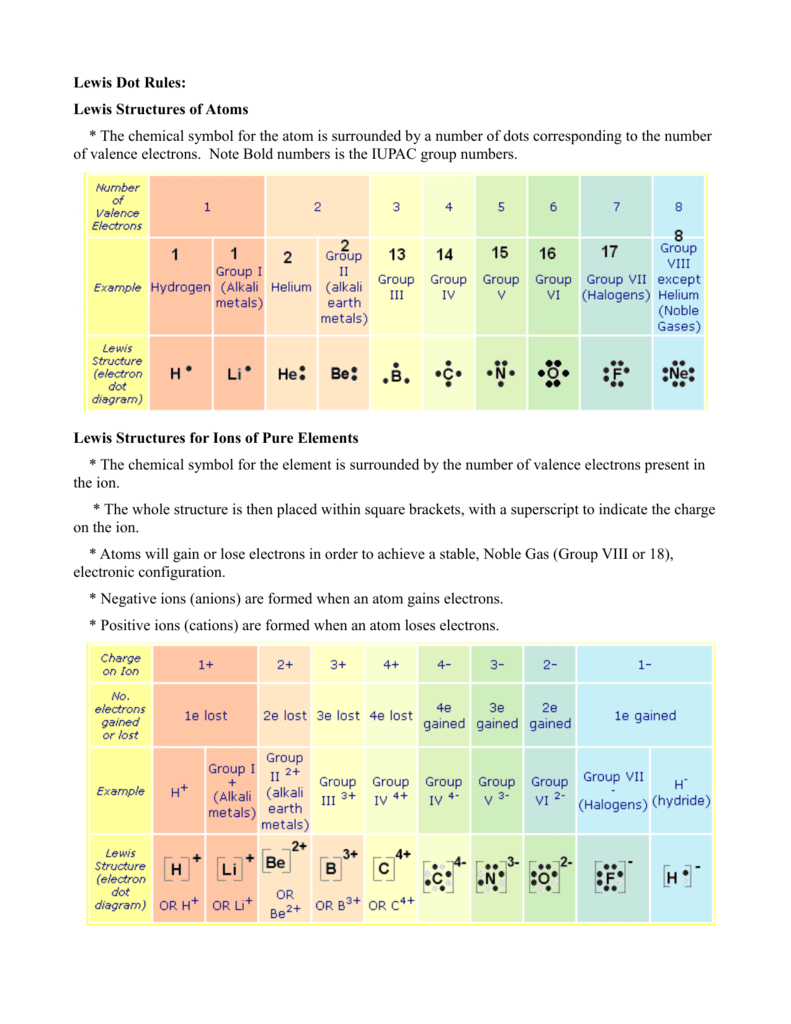
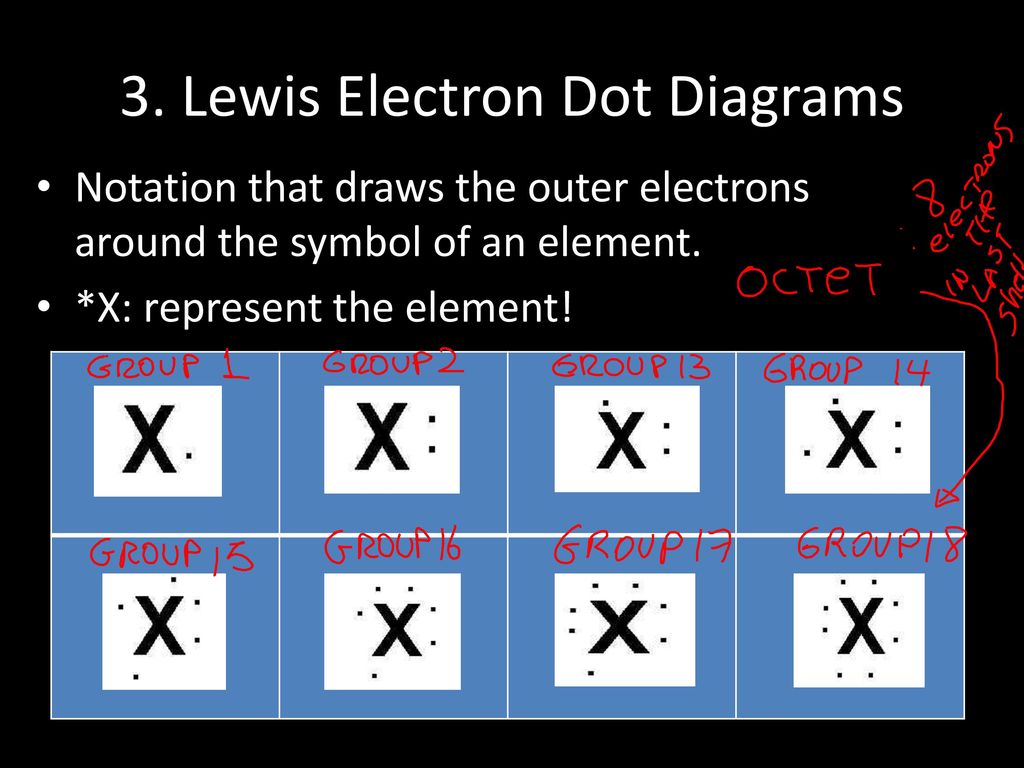
Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Lewis Dot Structures - Definition and Example | Chemistry Lewis structure is basically a graphic representation of the electron distribution around an atom. The major reason why learning Lewis dot structure is important is that it helps in predicting the number and type of bonds which can be formed around an atom. It also helps in predicting the geometry of the molecule.
What is a Lewis dot diagram? + Example - Socratic And thus Lewis dot treatment distributes the valence electrons. And we can easily find the number of valence electrons for a given atom by noting its Group number in the Periodic Table, which number gives required the number of electrons. For a simple example, consider ammonia, N H 3, the which has 5 nitrogen valence electrons, and 3 electrons ...
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired.
Lewis Structures (electron dot diagrams) Chemistry Tutorial Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students.
Lewis Dot Structures - YouTube Finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Those are lewis dot structures. Let's learn how...
The definition of electron dot diagram? - Answers The electron dot diagram is a way of working out the bonding of a molecule. The electron cloud is a description of the way that electrons surround the nucleus. People also asked Study Guides Stu's...
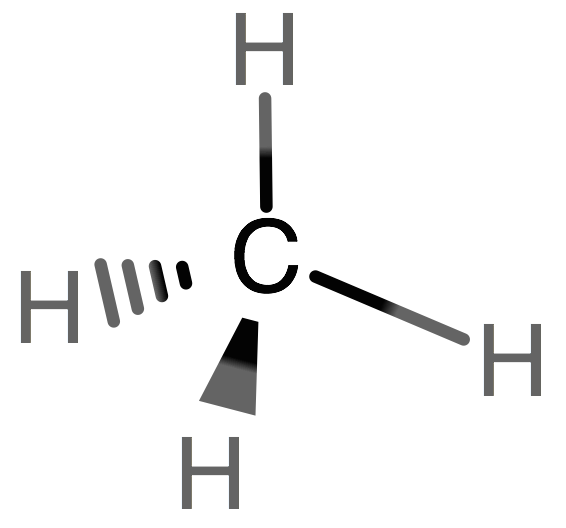
Carbon Lewis Dot Structure Definition | OrgoMadeSimple.com Lewis dot structure definition: a visual way to clearly depict the connection of atoms and the electrons present in a molecule. With a carbon Lewis dot structure, one can see how the atoms in a molecule are bonded together, which gives us more information about the structure than the molecular formula.
Electron dot diagram | Article about Electron dot diagram ... Electron dot diagram | Article about Electron dot diagram by The Free Dictionary Lewis structure (redirected from Electron dot diagram) Lewis structure [ ′lü·is ‚strək·chər] (chemistry) A structural formula in which electrons are represented by dots; two dots between atoms represent a covalent bond.
Radical (chemistry) - Wikipedia Delocalization effects can also be understood using molecular orbital theory as a lens, more specifically, by examining the intramolecular interaction of the unpaired electron with a donating group’s pair of electrons or the empty π* orbital of an electron-withdrawing group in the form of a molecular orbital diagram. The HOMO of a radical is ...
Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
What is the electron dot diagram for scandium ... Since the Lewis electron dot diagrams are based on the number of valence electrons, it would hold true that the elements in the same group would have the same electron dot diagram. What is an electron dot formula? Electron dot formula shows the number of valence electrons for that element with the help of dots.
Learn About Electron-Dot Structure | Chegg.com The electron dot structure is also known as the Lewis Structure. It is a simple way of representing the structure and bonding in a molecule along with the electrons in the valence shell. The bonding between the atoms in a molecule can also be explained by the electron dot structures.
42 boron lewis dot diagram - Modern Wiring Diagram Lewis structures,also called Lewis dot formulas or electron dot shapes (LEDs), are diagrams showing the bonding between atoms and the possible lone pairs of electrons within a molecule. Lewis structures can be drawn for any covalently bound molecule and coordination compounds. BF3 Lewis Structure, Molecular Geometry, and Hybridization BF3 Lewis ...
Na Lewis Dot Structure - ViralListClub.com A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Typically ionic Lewis dot structures include the ionic charge so the Na ion is labeled 1 and Cl is labeled -1. A single shared pair of electrons is ...
How to Calculate a Steric Number - Sciencing Feb 16, 2020 · The challenge in calculating the steric number is therefore less one of actual calculation and more of looking at the structure of the molecule in terms of bonding electrons and finding the two numbers you need. This is fairly easy to do if you look at the Lewis structure of the molecule and understand how to find a lone electron pair.
Definition of Lewis Structure | Chegg.com Now we want to thank first of all how many valence electrons each atom has We're going to need to use the periodic table to form a Lewis.structure or Louis line diagram. Now here I've shown an oxygen. In the Black we have six dots those represent six electrons.
Learn About Lewis Structure Of Ozone | Chegg.com Lewis structures are also known as Lewis electron dot structure, electron dot structure, dot structure, etc. The bonding by Lewis structure can be shown in a covalent and coordination molecule, where the bond is either formed by sharing of electrons or are coordinates. This theory of Lewis structure was introduced by Gilbert N. Lewis in 1916.
Electron Dot Diagram - YouTube The following lesson looks at drawing electron dot diagrams. Download the following lesson for free from iTunes by typing in the search window "PapaPodcasts"...
Lewis Structure Definition and Example - ThoughtCo A Lewis structure is a diagram that shows the covalent bonds and lone electron pairs in a molecule. Lewis structures are based on the octet rule. While Lewis structures are useful for describing chemical bonding, they are limited in that they do not account for aromaticity, nor do they accurately describe magnetic behavior. Definition
What is the Lewis electron-dot diagram for a fluoride ion? | Socratic January 7, 2017 - Fluorine is in Group 17 of the Periodic Table.................... And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). ...
Lewis Electron Dot Diagrams – Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Lewis Structures: Dot Symbols, Diagrams, Examples - Embibe A Lewis Structure or Electron Dot Structure is a very simplified representation of the valence shell electrons in a molecule. It denotes the way the valence electrons are arranged around the individual atoms in a molecule. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article "The Atom and the Molecule ."
PDF Electron Dot (Lewis) Diagrams - Mr. Sault's Classroom Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol.
Lewis dot structure definition July 26, 2004 - This site is dependent on its contributors. You don't want your hard work to be lost in the past. Make sure that you are given credit for your discoveries, so that others can reference you properly. Everyone knows the feeling when you are not given credit where credit is due.
Definition of Lewis formula (electron dot or Lewis structure) A double bond is represented by two pairs of dots, etc. Dots representing non-bonded outer-shell electrons are placed adjacent to the atoms with which they are associated, but not between the atoms. Formal charges (e.g. +, -, 2+, etc.) are attached to atoms to indicate the difference between ...
Tetrahedral in Molecular Geometry: Definition, Structure ... Oct 22, 2021 · Use the following Lewis diagram for ethyl amine to answer the questions: Remember that geometry refers to the geometry defined by the atoms, not the electron pairs. The geometry about atom 1 is ...
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.

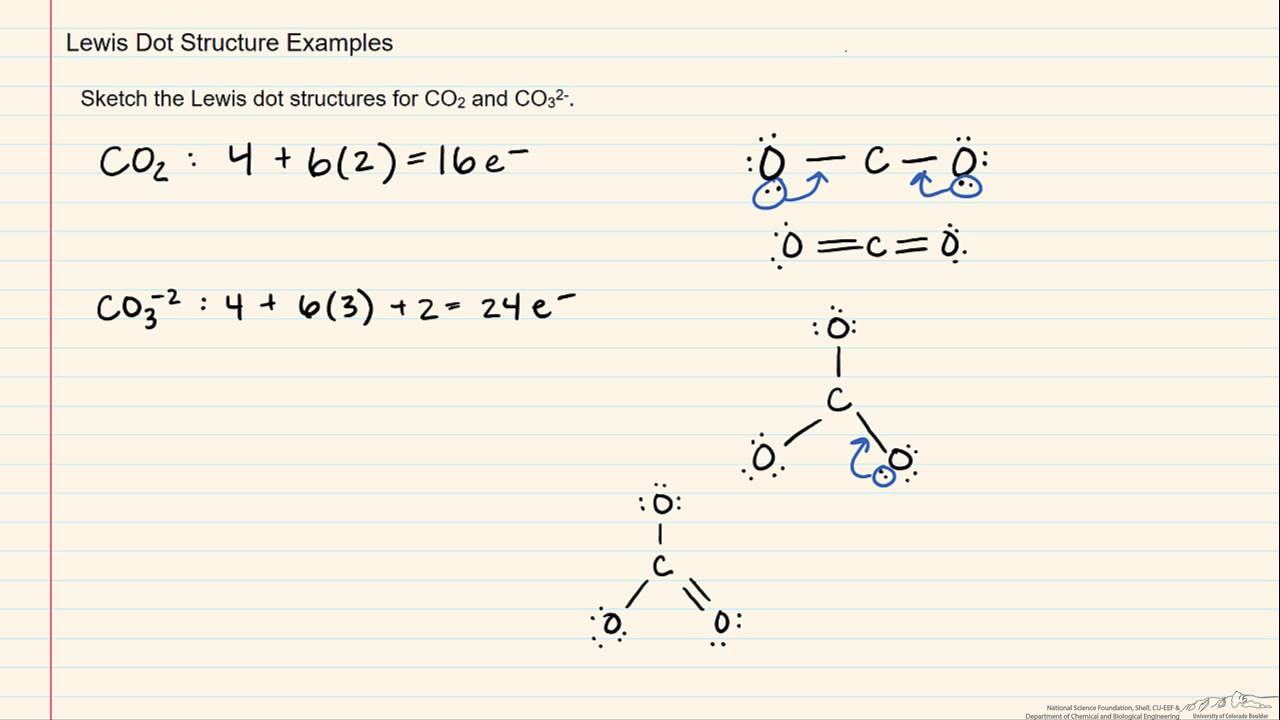

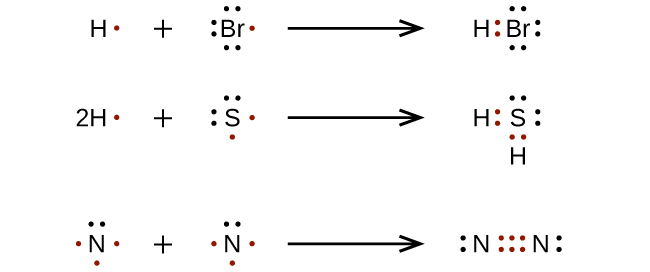


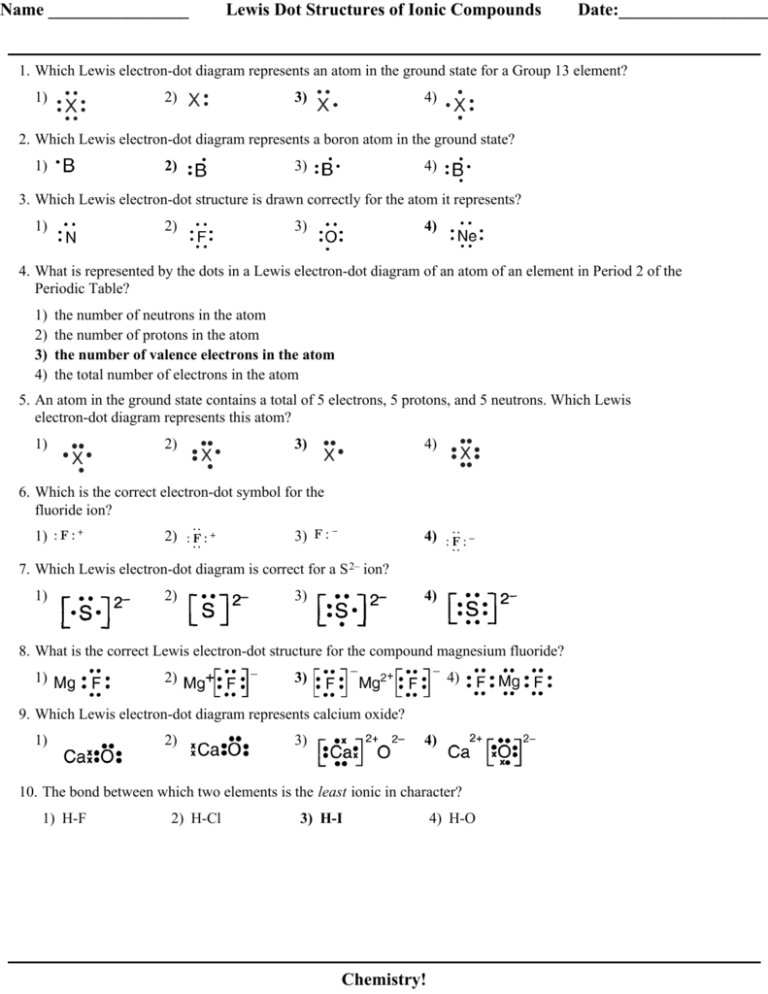













/lewisnitrite-56a128825f9b58b7d0bc90cf.jpg)


0 Response to "41 lewis electron dot diagram definition"
Post a Comment