41 dot diagram for nitrogen
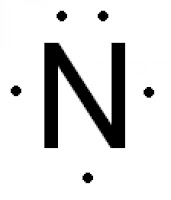
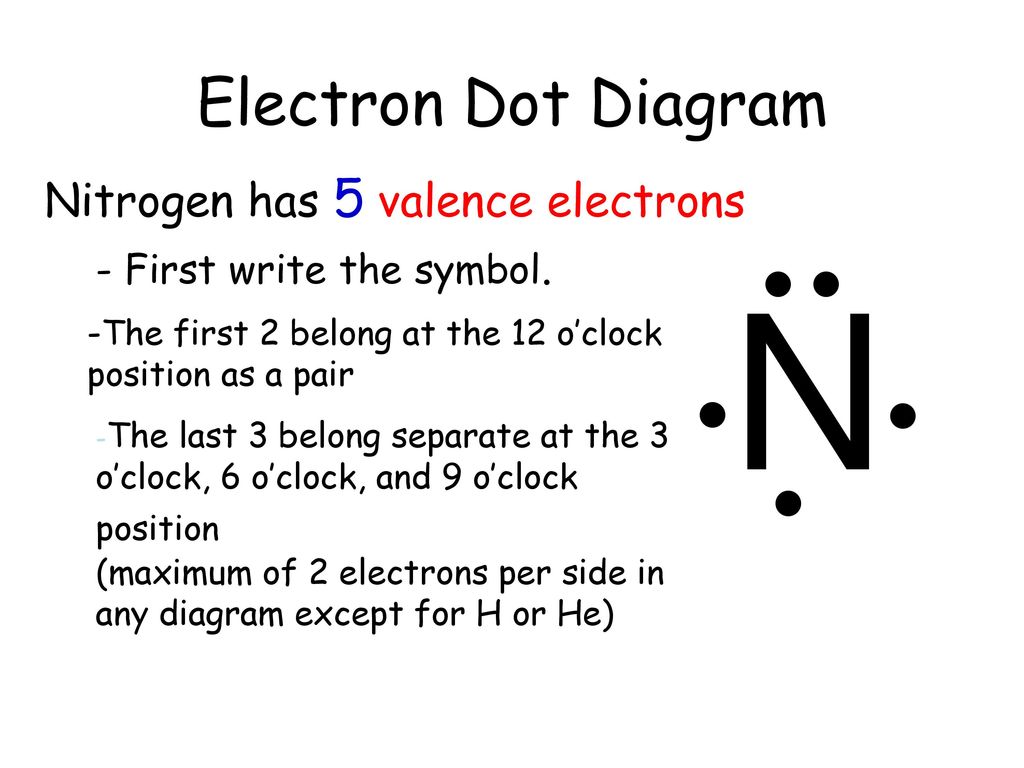
What is the electron dot diagram for nitrogen ... Which is the correct Lewis dot diagram for nitrogen? The five dot represent the five valence electrons of nitrogen. Next create a square around nitrogen, then place one of the valence electron on each side of the square, the last electron is placed beside any of the other electrons. Drawing dot- and- cross diagrams of Covalent Molecules - O ... Dot- and- cross diagram of covalent molecule (nitrogen) N2 Nitrogen is in Group V of the periodic table. This means that an atom of nitrogen has 5 valence electrons. In order to achieve stable octet configuration, nitrogen needs 5 valence electrons, in order words, 3 more electrons.
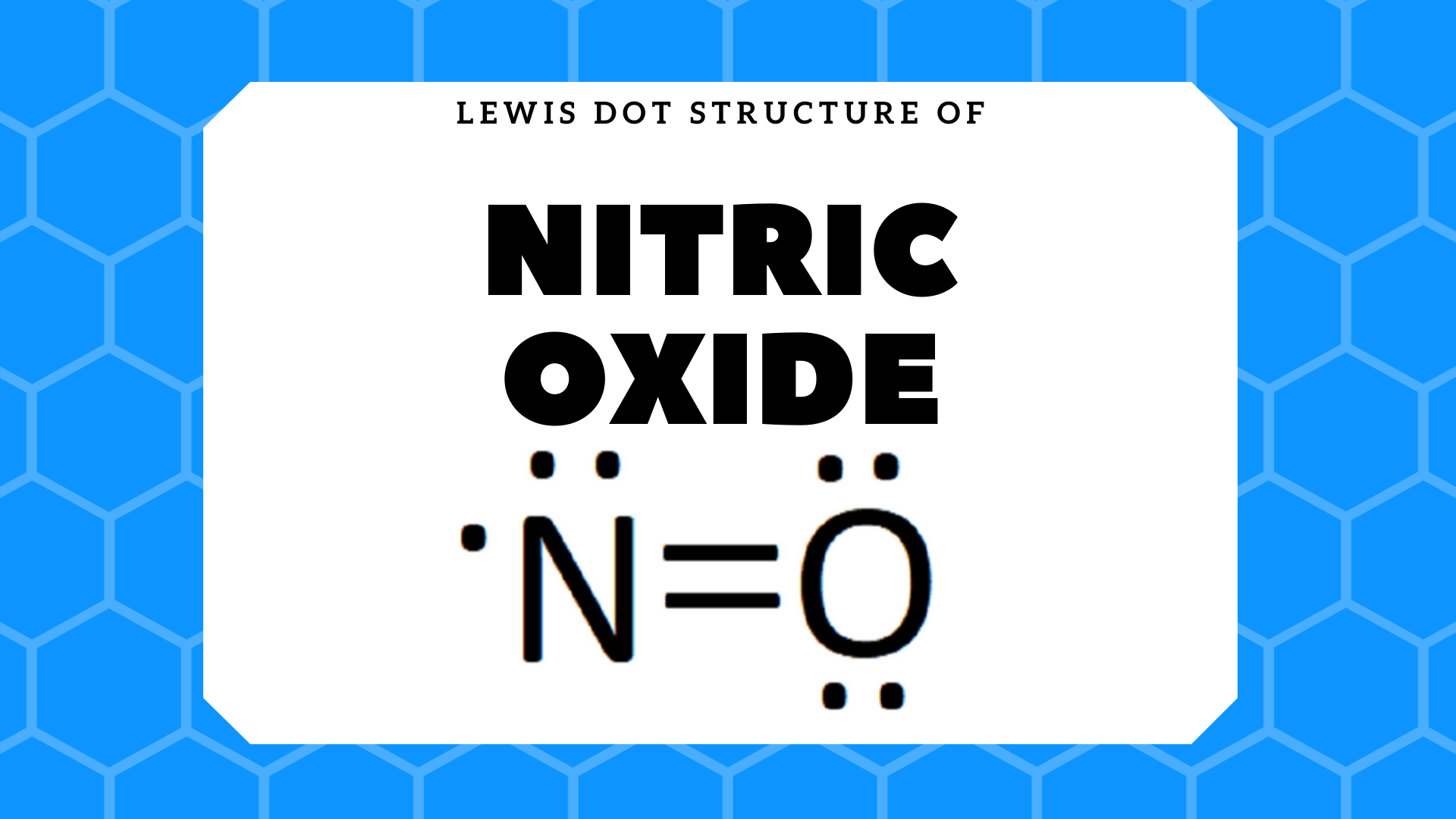
What is Lewis dot diagram of nitrogen gas? - Answers Nitrogen Monoxide is a paramagnetic gas, also known as nitric oxide.It is colorless and neutral.Its molecule orbital diagram resembles that of carbon monoxide. A Lewis symbol that has a non noble...

Dot diagram for nitrogen
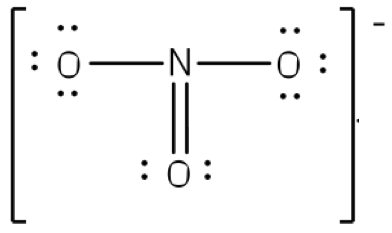
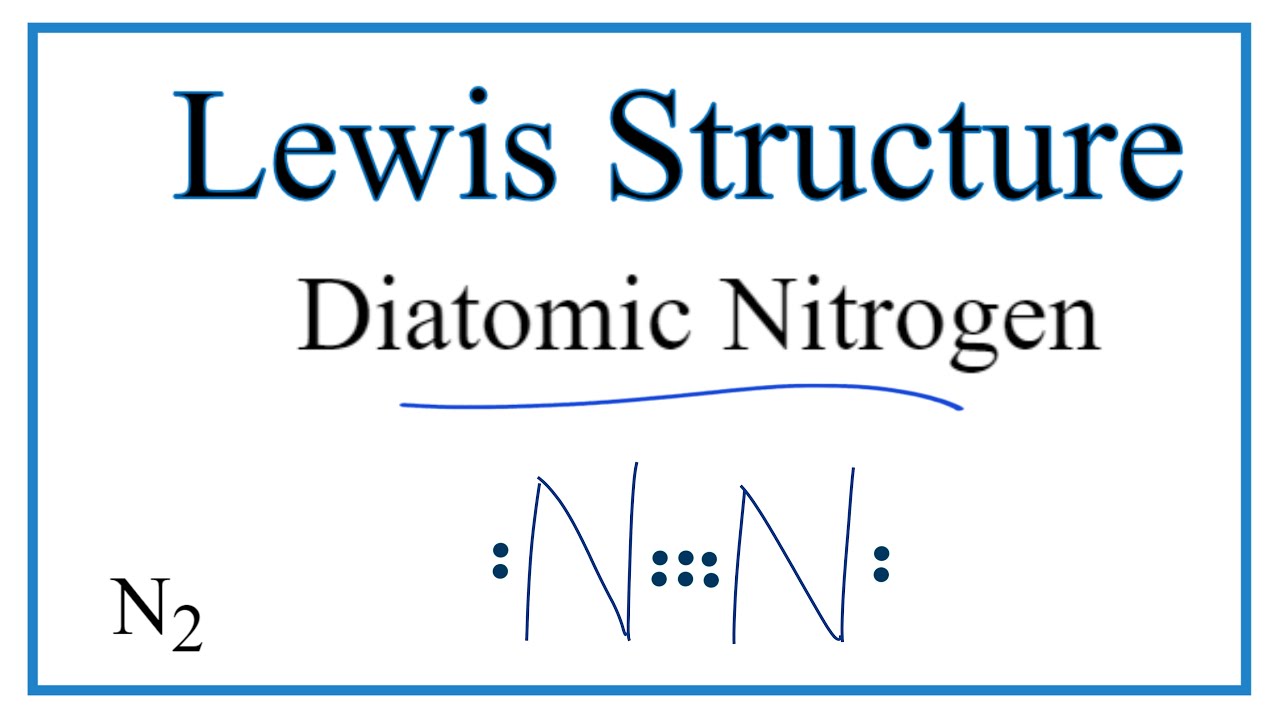
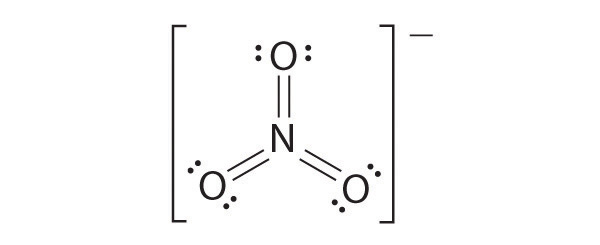
N2 Lewis Structure: Full Guide (2022 Updated) Nitrogen has five valence electrons in the N2 electron dot structure, classified as a group 5 on the periodic table. Align the two Nitrogens and then sandwich two valence electrons between them to make a chemical bond. There will be no center atom in the Lewis structure since both atoms have the same electronegativity. Electron Dot Diagrams | Chemistry for Non-Majors - Lumen ... Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... NO2 (Nitrogen Dioxide) Lewis Dot Structure - Science Trends Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.It is slightly toxic to humans, on account of its tendency to react in the human body and produce reactive species of nitrogen and ...
Dot diagram for nitrogen. %tiNitrogen Lewis Dot Structure:Drawing,Several Compounds ... Nitrogen dioxide lewis dot structure. Nitrogen is a 'group 15' element (electronic configuration: 1s2 2s2 2p3) and Oxygen is a 'group 16' element (electronic configuration: 1s2 2s2 2p4) in Periodic table. To draw the electron dot structure we count outer most shell electrons of the molecule. How to Draw the Lewis Dot Structure for N2: Nitrogen Gas ... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... How do you write/draw a Lewis structure for N? | Socratic 11 Apr 2016 — The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration of ...1 answer · Explanation: The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration ... What is the electron dot diagram for nitrogen? When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). Click to see full answer Also know, what are electron dot diagrams used for? There are shorthand ways to represent how atoms form covalent or ionic bonds.
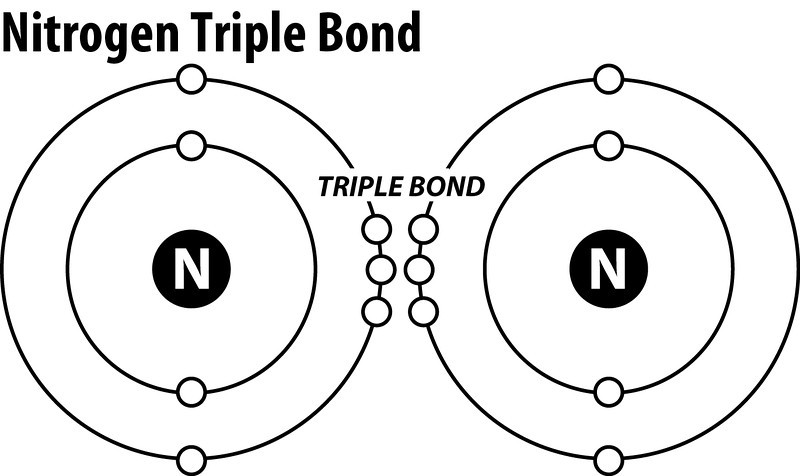
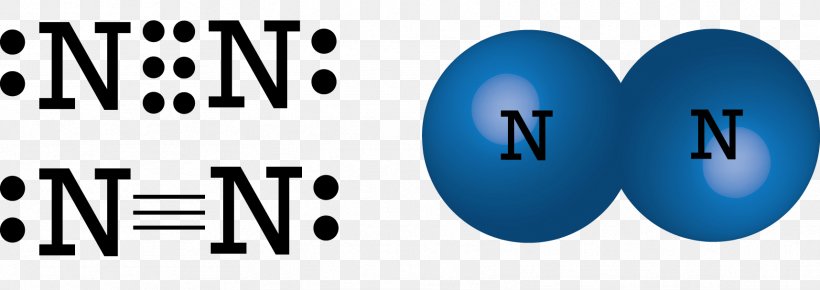
Draw the electron dot structure of : Nitrogen molecule (N=7] the question of the electron dot structure of Nitrogen molecule and is equal to 7 have to draw the molecular structure of Nitrogen nitrogen balance Shelby have 7 electronic pfi valence electrons of configuration is 25 so the valence electrons will be five similarly we will grow and other atoms of Nitrogen which will also have five electron-electron back karo electrons from each nitrogen khatm ... What is the Lewis dot structure for nitrogen ... What is the dot structure of NO2? The NO2 Lewis structure has a total of 17 valence electrons. It's not common to have an odd number of valence electrons in a Lewis structure. Because of this we'll try to get as close to an octet as we can on the central Nitrogen (N) atom. Electron Dot Diagram and Structural Formulas - Physics Forums Nitrogen Tri-iodide ( NI3 ) I know the basics of electron dot structures, but when the atoms start having double covalent bonds I lose track and get confused. Structural formulas are also unclear to me except for that they are a simpler version of the dot diagrams using a single line for single covalent bonds, double for double covalent, and so ... What is the Lewis dot structure for nitrogen gas ... What is the Lewis dot structure for nitrogen gas? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable.
Working with Laboratory Equipment - Prudent Practices in ... Working safely with hazardous chemicals requires proper use of laboratory equipment. Maintenance and regular inspection of laboratory equipment are essential parts of this activity. Many of the accidents that occur in the laboratory can be attributed to improper use or maintenance of laboratory equipment. This chapter discusses prudent practices for handling … NO2 Lewis Structure: Complete Guide (2022 Updated) NO2 contains three pairs of electrons surrounding the nitrogen atom for molecular structure. The one-electron allows the two connecting oxygen atoms to stretch out to a 134 angle. We use Lewis structure (Lewis dot structure) to understand the chemical bonds, the number of lone pairs, whether double or triple bonds, and how many electrons are in ... N2 Lewis Structure, Molecular Geometry, and Hybridization ... Since you have 2 atoms of Nitrogen, assign the valence electrons using dots in a diagram to each atom-like 5 dots around each atom. Use symbol N to represent the atom. Both the atoms have the same electronegativity, there will be no central atom in the structure. Parts of a Draft Beer System & How They Work [Diagram] Glycol Cooled Draft System. If the kegs cannot be kept refrigerated within close proximity to the draft tower and faucets, then a long draw draft system is required. A glycol cooled draft system is a long draw system that uses a glycol chiller or power pack to pump a mixture of glycol and water through a trunk line that keeps draft beer at a consistent temperature as it travels from keg to tap.
lewis dot diagram Quiz - Quizizz This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
42 lewis dot diagram for nitrogen - Wiring Diagrams Manual What is the electron dot diagram for nitrogen? Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N).
Pearson Edexcel Certificate Pearson Edexcel International ... 19-05-2016 · 1 The diagram shows a kettle of boiling water. water droplets ... The two main gases present are the elements nitrogen and oxygen. (a) Another element that is present in air is (1) A argon B carbon dioxide ... Draw a dot and cross diagram …
This is a correct dot diagram for nitrogen (N) This is a correct dot diagram for magnesium (Mg) This could be the dot diagram of ; Nitrogen forms compounds with the elements hydrogen, oxygen, magnesium and sodium.These compound have the formule NQ2, X3N, Y3N2 and NZ3, where Z represents nitrogen.What are the identities of Q, X, Y and Z
Nitrogen Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of a Nitrogen atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Nitrogen, we got to know, it has 5 valence electrons. So, just represent these 5 valence electrons around the Nitrogen atom as a dot. The electron configuration of Nitrogen
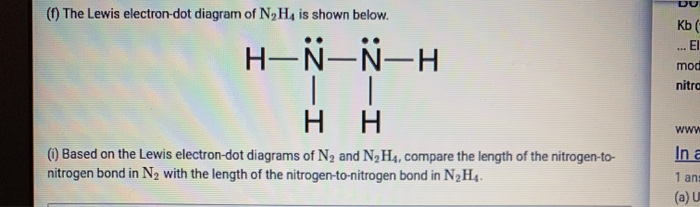
Dot and cross diagrams for larger diagrams. This dot and cross diagram shows the outer shells touching. This method is easiest for single covalent bonds as there is not much room where the atoms' shells touch for drawing lots of electron pairs. Nitroxyl contains a single covalent bond between hydrogen and nitrogen and a double covalent bond between nitrogen and ...
Electron Dot Diagram For Nh3 - schematron.org Nitrogen goes in the centre. Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides.
Lewis Dot Diagram For N2 - schematron.org There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . The Lewis Structure for N2 looks easy at first.
Atomic numbers and electron configurations ... - Quizlet Dot structures make it easy to count electrons and they show the number of electrons in each electron shell. Arrow and line diagrams show the spin of electrons and show every orbital. Written configurations require minimal space and show the distribution of electrons between subshells.
Nitrogen trichloride (NCl3) lewis dot structure, molecular ... As per the NCl3 lewis dot structure, nitrogen is the central atom that has 3 bonded pairs of electrons and one lone present on it. Hence the formula of NCl3 becomes AX3N1 So, according to the VSEPR chart, if the molecule has the formula of AX3N1 then the molecule shape of that molecule is trigonal pyramidal, and electron geometry is tetrahedral.
Lewis dot of Nitrogen Monoxide (NO) | Chemistry Net what is the Lewis structure of NO nitrogen monoxide, Lewis structures of nitrogen monoxide, Lewis electron dot structures of nitrogen monoxide, electron dot structures of nitrogen monoxide, NO Lewis structures, NO electron dot structures, NO dot structures, pi an d, for the draw, lewis no, dot structure of NO, electron dot lewis structure of NO, resonance structures of NO, ap chemistry lewis ...
What is the electron dot diagram for Helium? - Qaalot Consequently, what is the Lewis dot diagram for Helium? The Lewis image for helium: Helium is considered one of the noble gases and accommodates a full valence shell. Not like the different noble gases in Group 8, Helium solely accommodates two valence electrons. In the Lewis image, the electrons are depicted as two lone pair dots.
Compare the Lewis dot structure of nitrogen and phosphorus ... Compare the Lewis dot structure of nitrogen and phosphorus and explain why you might expect these two atoms to exhibit similar bonding properties. Name one element that you would expect to exhibit bonding properties similar to boron. Explain.-----1.5 | Draw a Lewis dot structure for each of the following atoms: a) carbon b) oxygen c) Fluorine d) Hydrogen e) Bromine f) Sulfur g) Chlorine h ...
Lewis Dot Structure for Nitrogen Atom (N) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...
which lewis electron-dot diagram represents a nitrogen ... which lewis electron-dot diagram represents a nitrogen atom in the ground state which lewis electron-dot diagram represents a nitrogen atom in the ground state Answer To do this, you 1st figure out the atomic number of which ever element you are working on. In this case, it's Nitrogen. Nitrogen has an atomic number of 7.
What intermolecular forces are present in CO_2? | Socratic May 06, 2018 · Dispersion Forces CO_2 has dispersion forces or van der waals forces as its only intermolecular force. Since CO_2 is made of one carbon and 2 oxygen and both carbon and oxygen are non-metals, it also have covalent bonds. For extra information, there are 3 types of intermolecular forces. Dispersion Forces Dipole-dipole Hydrogen bonds Dispersion forces are weaker than dipole-dipole and dipole ...
Exceptions to the Octet Rule | Boundless Chemistry The rule is applicable to the main- group elements, especially carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium and magnesium. Valence electrons can be counted using a Lewis electron dot diagram. In carbon dioxide, for example, each oxygen shares four electrons with the central carbon.
Draw the electron dot structure of Nitrogen molecule [N = 7] Nitrogen atom shares three electrons forming a triple covalent bond. solution. expand. Solve any question of Chemical Bonding and Molecular Structure with:-.1 answer · Top answer: Nitrogen molecule N = 7 & 2 & 5 & K & L N = 7 & 2 & 5 & K & L Nitrogen atom shares three electrons forming a triple covalent bond.
Draw the electron dot structure of Nitrogen molecule [N = 7] N=7⇒. . 2K. . 5L. . Nitrogen atom shares three electrons forming a triple covalent bond. Solve any question of Chemical Bonding and Molecular Structure with:-. Patterns of problems.
NO2 (Nitrogen Dioxide) Lewis Dot Structure - Science Trends Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.It is slightly toxic to humans, on account of its tendency to react in the human body and produce reactive species of nitrogen and ...
Electron Dot Diagrams | Chemistry for Non-Majors - Lumen ... Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
N2 Lewis Structure: Full Guide (2022 Updated) Nitrogen has five valence electrons in the N2 electron dot structure, classified as a group 5 on the periodic table. Align the two Nitrogens and then sandwich two valence electrons between them to make a chemical bond. There will be no center atom in the Lewis structure since both atoms have the same electronegativity.
:max_bytes(150000):strip_icc()/NO2_Dot-56a12a2c3df78cf772680359.png)



![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1890007_1909574_ans_16e2a124f2974b5694de1a9f3c97eebd.png)







![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1648865_1784763_ans_65460fbc5eda4a3b8021b75bbc2803a5.png)










![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1890011_1909650_ans_86e4b88529e541639e84039753e5b955.png)
0 Response to "41 dot diagram for nitrogen"
Post a Comment