40 lewis dot diagram for co
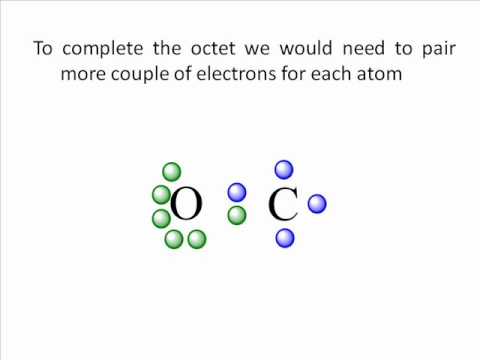
CO Lewis Structure: How to Draw the Dot Structure for CO ... Drawing the Lewis Structure for CO (Carbon monoxide) Viewing Notes: CO is a clear, odorless, poisonous gas. It can be formed by combustion in oxygen poor environments and leads to a number of human deaths each year. How to Draw the Lewis Dot Diagram for Carbon monoxide (CO ... te them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of CO structure there are a total of 10 valence...
Easy Lewis Dot Structure Worksheet - Worksheet Kids News Co 3 2 4. These worksheets have students explore the nature of atoms and their structure. Lewis structure displaying top 8 worksheets found for this concept. Some of the worksheets for this concept are lewis electron dot structure answers electron dot diagram work with answers lewis dot practice work with answers 5 11 electron diagrams and ...

Lewis dot diagram for co
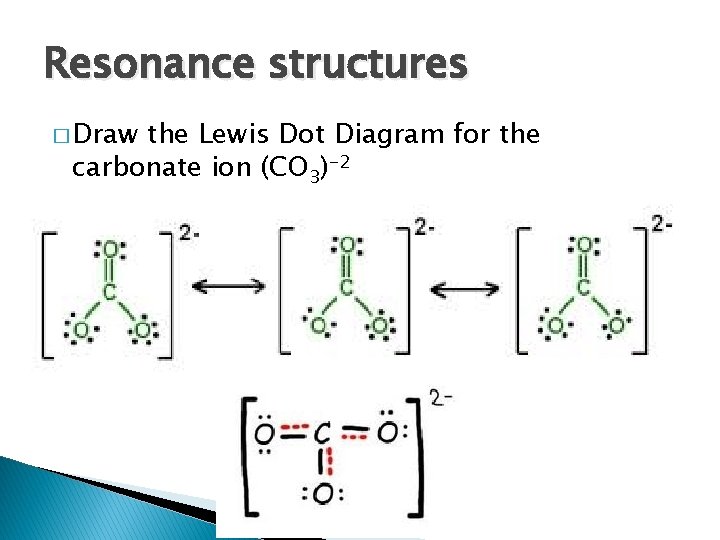
H2O2 lewis structure, molecular geometry, hybridization, bond ... The H2O2 lewis dot structure is very simple and the procedure for drawing it same as the other molecules. Let’s see how to draw this step by step. Follow some steps to drawing the H2O2 Lewis dot structure 1. Count total valence electron in H2O2. In the first step, we need to calculate how many valence electrons are present in it. PPTX Topic: Lewis Dot Diagrams for Ionic Compounds Topic: Lewis Dot Diagrams for Ionic Compounds. Do Now: Identify ionic compounds. CO. 2 MgCl. 2 NH. 4. ClNaOH. NH. 3 CH. 4 CuSO. 4 HF. Magnesium's outer shell is now empty. Fluorine's outer shell is now full. Lewis Diagrams for Ionic Compounds. NaCl's Lewis structure: PDF Lewis Dot Structures Pogil Key - Hudson City School District Complete the Lewis dot structure for IN2 Complete the Lewis dot structure for C02 10. Complete the Lewis dot structure for CO 11. Complete the Lewis dot structure for HCN STOP Your group will check your answers with the instructor before moving on. Model V: Resonance Structures— When One Lewis Structure Isn't Enough Read This!
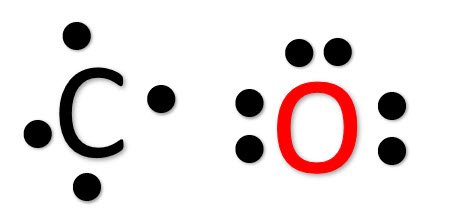
Lewis dot diagram for co. Lewis Structure Definition and Example - ThoughtCo Oct 16, 2019 · Lewis structures go by many names, including Lewis electron dot structures, Lewis dot diagrams, and electron dot structures. All these names refer to the same sort of diagram, which is intended to show the locations of bonds and electron pairs. CO2 Lewis Structure (2021 UPDATED) All You Need To Know Dec 22, 2021 · In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2. Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure of CO (Carbon Monoxide) A carbon monoxide molecule consists of one carbon atom and one oxygen atom. The carbon atom requires four electrons to obtain octet configuration whereas the oxygen atom requires two. Therefore, the valency is satisfied via the donation of a lone pair of electrons for bonding by the oxygen atom. PDF Lecture B1 Lewis Dot Structures and Covalent Bonding Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
Lewis structure calculator | Lewis structure generator Lewis structure definition | What is a Lewis dot diagram? The formation of a chemical bond (ionic and covalent) involves the transfer of electrons or the exchange of electrons. Lewis structures can be used to represent valence shell electrons in a chemical bond. CO2 Lewis Structure | Lewis Dot Structure For Molecules ... Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure works on the octet rule, which means that all the atoms would have eight electrons in their valence shell except hydrogen. Carbon dioxide (CO2) lewis dot structure, molecular geometry ... In the CO2 lewis structure, there are a total of 4 lone pairs means 8 nonbonding electrons are present. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Let’s see how to draw a CO2 lewis dot structure with simple steps. Follow some steps for drawing the Lewis dot structure for CO2 1. 38 which lewis electron-dot diagram represents a molecule ... In the box below, draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PC13 (c) ammonia 60) 61) 62) 65) In the box provided, draw a Lewis electron-dot diagram for a molecule of chlorine, Ch. in terms of electrons, why the bonding in NaCl is Ionic.
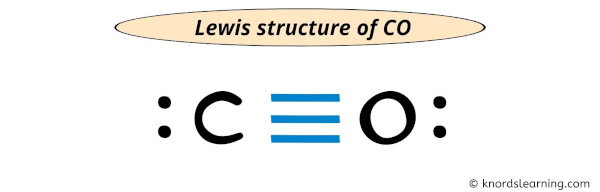
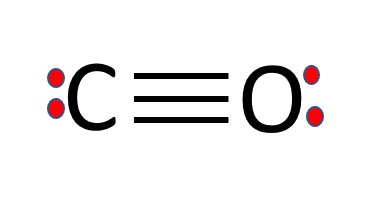
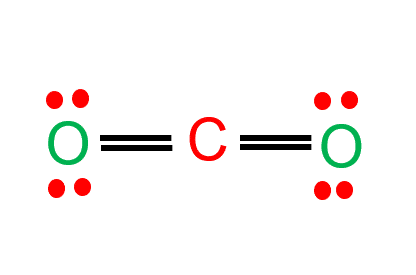
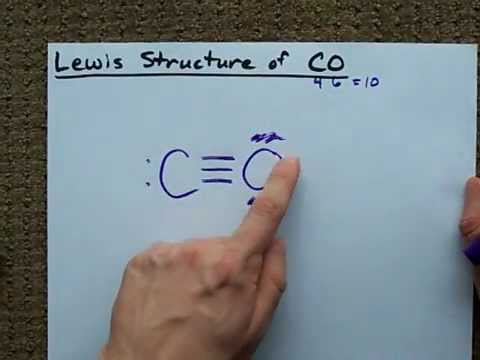
CO Lewis Structure - Lewis Dot Structure | Chem Helps Lewis Structure for CO is shown with a triple bond between carbon and oxygen atoms and 2 dots for each atom to show valence electrons. How many bonding electrons are in the lewis structure of carbon monoxide, co? Carbon monoxide is formed with 1 carbon and 1 oxygen. These atoms form a triple bond between them to create carbon monoxide molecule. CO Lewis Structure, Geometry, and Hybridization ... CO Lewis Structure, Geometry, and Hybridization. Carbon monoxide (CO) is a tasteless and odorless flammable gas that is quite toxic in nature to the fauna. It is so because, carbon monoxide uses hemoglobin, an oxygen carrier, to reach throughout the body when in a concentration of more than 35ppm. The carbon monoxide is produced from the ... Carbon monoxide (CO) Molecule Lewis Structure CO lewis structure. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. However, oxygen atoms has a +1 charge and carbon atom has a +1 charge. In next sections, we will draw CO lewis structure step by step. Steps of drawing lewis structure of CO molecule. There are guidelines (several steps) to ... Write the Lewis dot structure of CO molecule. The lewis dot structure of carbon monoxide is: :C≡O: Solve any question of Chemical Bonding and Molecular Structure with:-.
Compounds and Mixtures - BrainPop Learn the difference between a compound and a mixture. Which is more difficult to undo, and where does sea water and salad dressing come into it?
draw the lewis dot structure for co | How to Draw the ... draw the lewis dot structure for co | draw the lewis dot structure for co | draw the lewis dot structure for co2 | draw the lewis dot structure for co2−3 | dr
Ap Chemistry Lewis Dot Structure Worksheet - Chemistry ... Mole calculations worksheet 2. Lewis Dot Structures Jun 07 2011 Drawing Atoms in a Cross Shape Part 3 Filling in the Valence Electrons of an Electron Dot Structure Lewis Structure Part 4 Filling in the Valence Covalent dot and cross worksheet Chemistry unit 10 worksheet 3 answers.
Lewis Structure of CO (Carbon Monoxide) - YouTube The Lewis Structure (Lewis Dot Diagram) for CO.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill oute...
Lewis Structures: Single, Double & Triple Bonds - Video ... Nov 21, 2021 · Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows the valence electrons in an element ...
What do the dots on an electron dot diagram represent ... What is the Lewis dot structure for carbon monoxide? The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule.
Draw a lewis dot structure for Agcl and Co(NO3)2 - bartleby 8.1 Chemical Bond Formation And Lewis Electron Dot Structures 8.2 Covalent Bonding And Lewis Structures 8.3 Atom Formal Charges In Covalent Molecules And Ions 8.4 Resonance 8.5 Exceptions To The Octet Rule 8.6 Molecular Shapes 8.7 Electronegativity And Bond Polarity 8.8 Molecular Polarity 8.9 Bond Properties: Order, Length, And Dissociation ...
Lewis Structure Worksheet - Jojo Worksheet Lewis structure worksheet 1 answers lewis structure expanded octet co lewis structure lewis structure tool. For those of you that enjoy such things some more Lewis structures to draw. Electron Dot Lewis Structures A Lewis or Electron Dot Structure is a convenient representation of the valence electrons in an atom.
Draw the Lewis structure for CO - Ask4Essay A step-by-step explanation of how to draw the Lewis Dot Diagram for CO. Use the periodic table of elements to find the total number of valence electrons for the CO molecule. Once we know how many valence electrons does carbon monoxide have we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Lewis Dot Rules | Grandinetti Group Draw the Lewis dot structure for CO. The number of valence electrons is 4 + 6 = 10 electrons or 5 pairs. Since both C and O allow multiple bonds we can still follow the octet and write: If there is not enough electrons to follow the octet rule, then the least electronegative atom is left short of electrons. Draw the Lewis dot structure for BeF2.
CO32- Lewis Structure, Molecular Geometry, Hybridization, and ... Mar 24, 2021 · Lewis Structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons. Hence, Lewis Structure is also commonly called Electron Dot Structure. Let us proceed to draw the most appropriate LS diagram of CO32- ion. Step 1: Count the Total Number of Valence Electrons.
Lewis Structures: Learn How to Draw Lewis Structures ... How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16 Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
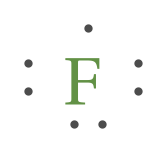
Lewis Structures: Dot Symbols, Diagrams, Examples, Questions What is Lewis Dot Structure? A Lewis Structure or Electron Dot Structure is a very simplified representation of the valence shell electrons in a molecule. It denotes the way the valence electrons are arranged around the individual atoms in a molecule. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his \(1916 ...
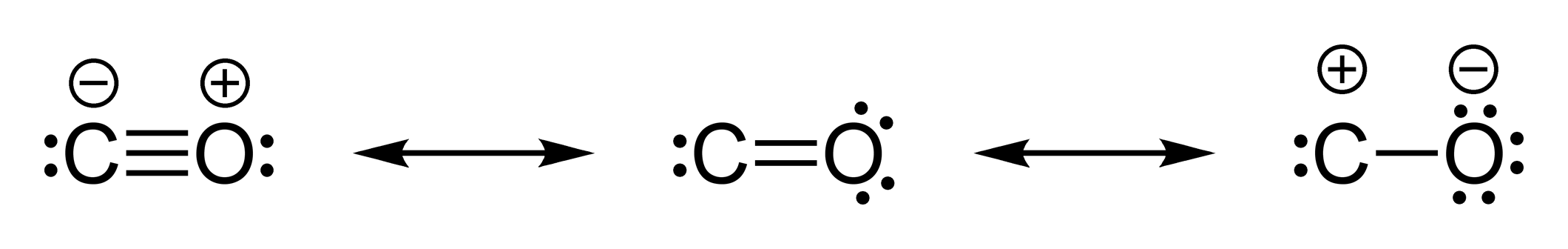
What is the Lewis structure of CO? | Socratic What is the Lewis structure of CO? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Truong-Son N. Oct 30, 2015 This often looks wrong to a student who is used to seeing double bonds on oxygen. Students are typically taught an electron-counting method, which goes as follows: ...
How to Draw CO Lewis Structure - Lewis Dot Structure ... But the CO Lewis Structure which shown above is still unstable because octal of C atom is not completed. Another lone pair of O atom should be converted to another bond. So, we can achieve a triple bond between C and O atoms. But, +1 charge on O and -1 charge on C will remain. Check out the final CO Lewis Dot Structure below: CO Lewis Dot Structure
How To Draw A Lewis Dot Structure For Co2 ... What Is The Lewis Diagram For Co2? The Lewis structure of carbon dioxide (CO) consists of two oxygen atoms and one carbon atom. The carbon atom in the CO has two double bonds. The valence shells of carbon atoms and oxygen atoms each contain two lone pairs. Linearity is the characteristic of CO.
Lewis Structure for CO - UMD The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. How to Draw the Lewis Dot Structure for Carbon monoxide It is helpful if you:
Write the Lewis dot structure of CO molecule class 11 ... Let us now write the electron dot structure also called as Lewis dot structure for C O molecule. - Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule.
PDF Lewis Dot Structures Pogil Key - Hudson City School District Complete the Lewis dot structure for IN2 Complete the Lewis dot structure for C02 10. Complete the Lewis dot structure for CO 11. Complete the Lewis dot structure for HCN STOP Your group will check your answers with the instructor before moving on. Model V: Resonance Structures— When One Lewis Structure Isn't Enough Read This!
PPTX Topic: Lewis Dot Diagrams for Ionic Compounds Topic: Lewis Dot Diagrams for Ionic Compounds. Do Now: Identify ionic compounds. CO. 2 MgCl. 2 NH. 4. ClNaOH. NH. 3 CH. 4 CuSO. 4 HF. Magnesium's outer shell is now empty. Fluorine's outer shell is now full. Lewis Diagrams for Ionic Compounds. NaCl's Lewis structure:
H2O2 lewis structure, molecular geometry, hybridization, bond ... The H2O2 lewis dot structure is very simple and the procedure for drawing it same as the other molecules. Let’s see how to draw this step by step. Follow some steps to drawing the H2O2 Lewis dot structure 1. Count total valence electron in H2O2. In the first step, we need to calculate how many valence electrons are present in it.






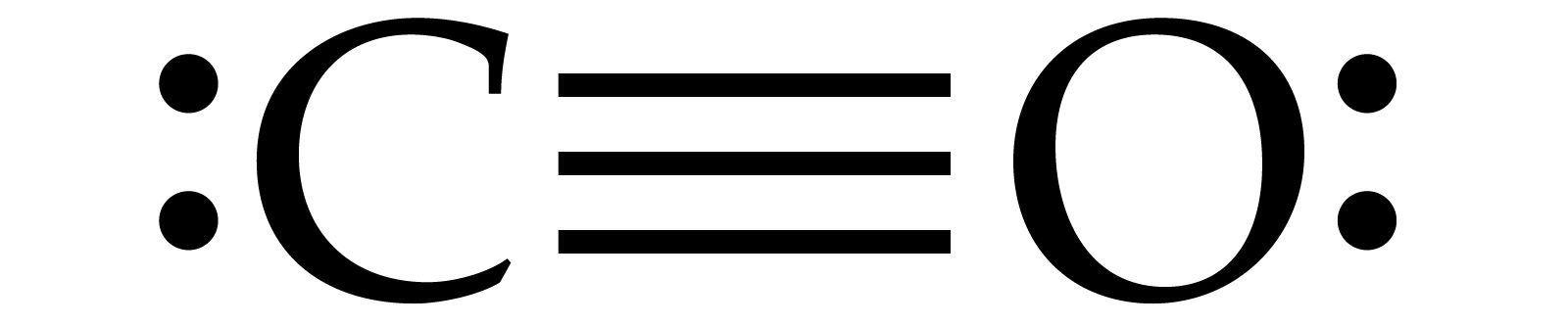


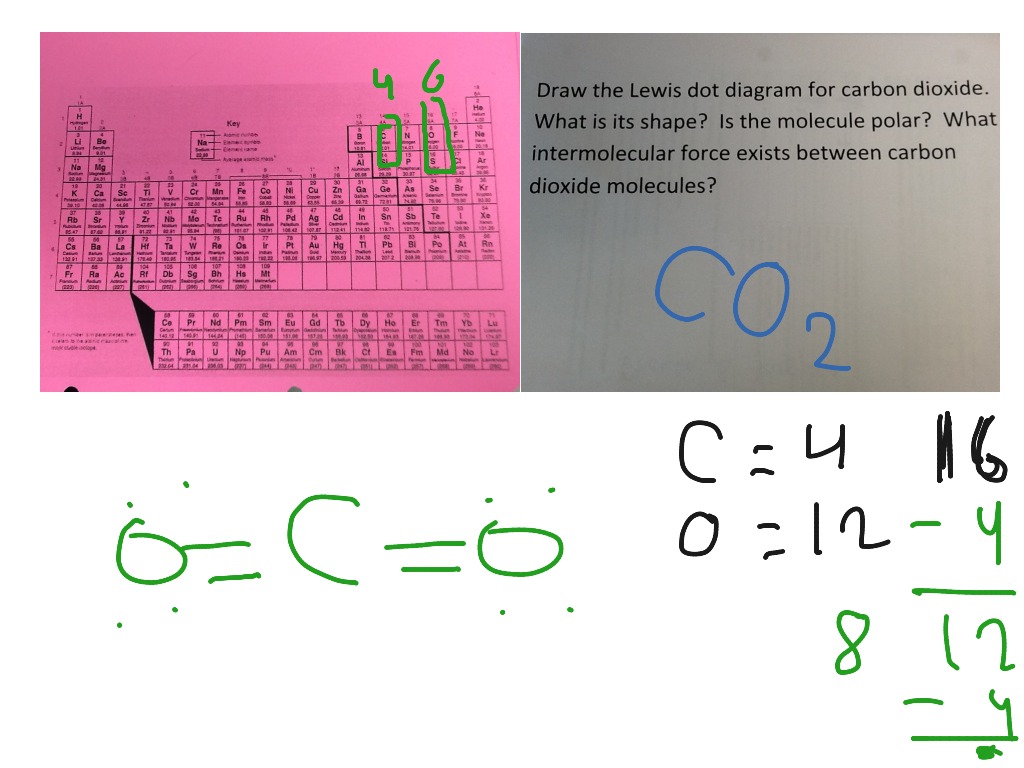











/lewisnitrite-56a128825f9b58b7d0bc90cf.jpg)

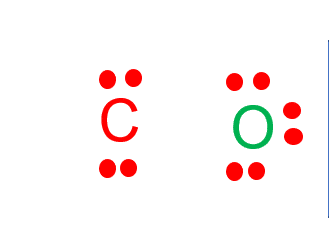
0 Response to "40 lewis dot diagram for co"
Post a Comment