42 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?
Refer to the following Lewis dot diagrams to answer this Question. 5. 6. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? • C • 2: hydrogen carbon 9. 10 A B 21 12 13 :0: :F : 15 16 oxygen flourine hp BADU. ∴ (14 - 2) = 12 valence electron. Now we are left with 12 valence electrons more. 4. Place remaining valence electrons starting from outer atom first. As Cl2 lewis's structure only contain two atoms that are similar so you can assume any of one is central and the other one is the outer atom.
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? When electrons are added to the outermost shell of a carbon atom, it forms The electrons that occupy the outermost filled shell are called Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond

Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?
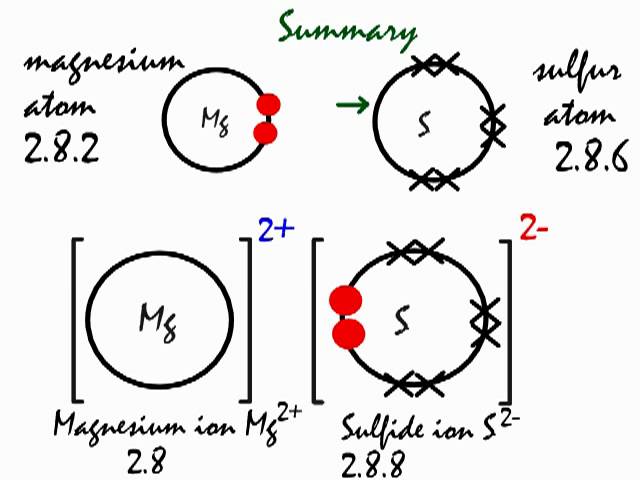
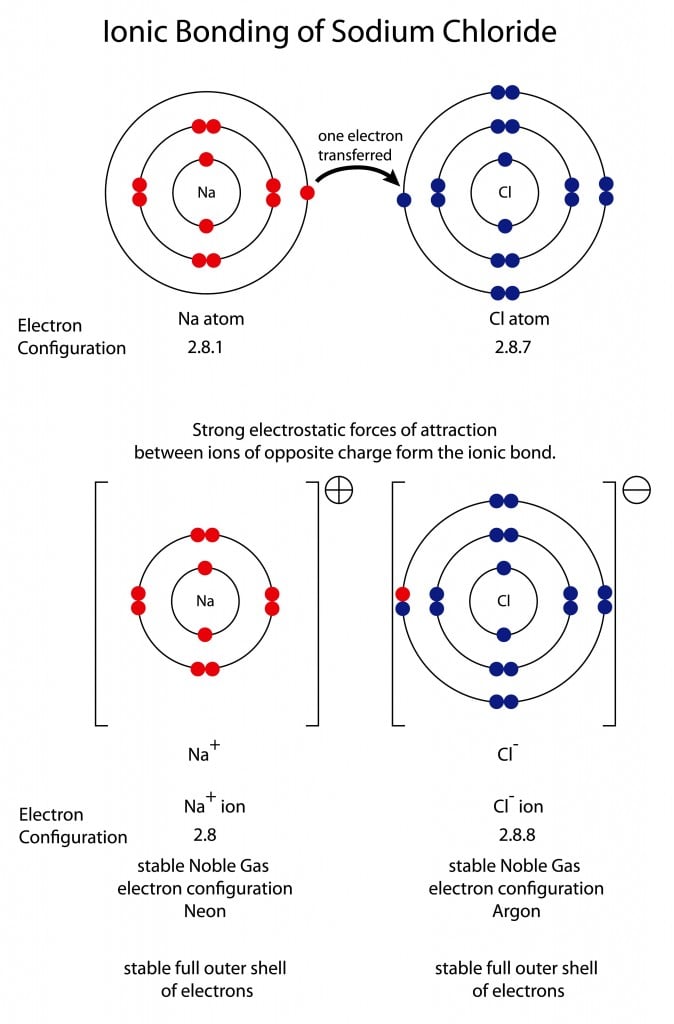
The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (In table salt, this electron comes from the sodium atom.) \[\ce{e^{-} +Cl -> Cl^{-}}\] In this case, the ion has the same outermost shell as the original atom, but now that shell has eight ... Which Lewis dot diagram shows an atom that needs two more electrons in its outermost shell? Related Answer. Using electron-dot diagrams which show only the ...
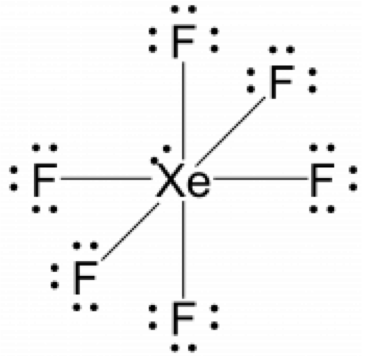
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?. Apr 25, 2017 · Atomic number of oxygen is 8 and its electronic distribution is 2, 6. In order to complete octet, oxygen needs 2 more electrons. Atomic number of fluorine is 9 and its electronic distribution is 2, 7. In order to complete octet, fluorine needs 1 more electron. Hence, we can conclude that out of the given options, lewis dot structure of oxygen atom needs 2 more electrons in its outermost shell. Play this game to review Chemistry. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Preview this quiz on Quizizz. ... Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Unit 7 Test Review DRAFT. 10th grade. 11 times. Chemistry. 63% average accuracy. 5 months ago ... To write a Lewis symbol for an atom, place the atom's chemical symbol in the center. This symbol will be the atomic core and will represent the nucleus and inner electrons for that atom. The valence electrons will be represented as a dot. Dots are placed around the atomic core singly for the first four electrons. Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom:
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. A Lewis symbol is a representation of an atom with its valence electrons. It shows the chemical symbol of the atom surrounded by dots which symbolize the valence electrons. ... such as in Ch, can be illustrated using the Lewis dot diagram. Notice that one pair of bonding electrons (i.e., single dash) is equivalent to one covalent bond, and that ... 30 Questions Show answers. Q. How many electrons should Oxygen have around its Lewis dot model? Which of the following shows a correct Lewis dot structure? Which element could X represent? Q. How many electrons should Carbon have around its Lewis dot model? Q. According to the octet rule most elements need _______ valence electrons. Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom:
Terms in this set (18) Which Lewis dot diagram shows an atom that needs 2 more electrons in it's outermost shell? Oxygen Which type of energy is the energy that it takes to remove an electron from it's shell? Ionization How does the Periodic Table organize atoms of elements with the same number of valence electrons? in columns The central atom needs to be with less electronegative value in the Lewis dot structure so that it can share more electrons with its surrounding atoms. Being an earth alkaline metal, beryllium is less electronegative than iodine in BeI2. Hence, put Be as the central atom and two iodine atoms as surrounding atoms. 2. Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol. Mar 14, 2018 · Oxygen is reactive because it needs to gain two more electrons to fill its outermost shell while neon's outermost shell is completely full of electrons. In more technical terms, neon has an octet...
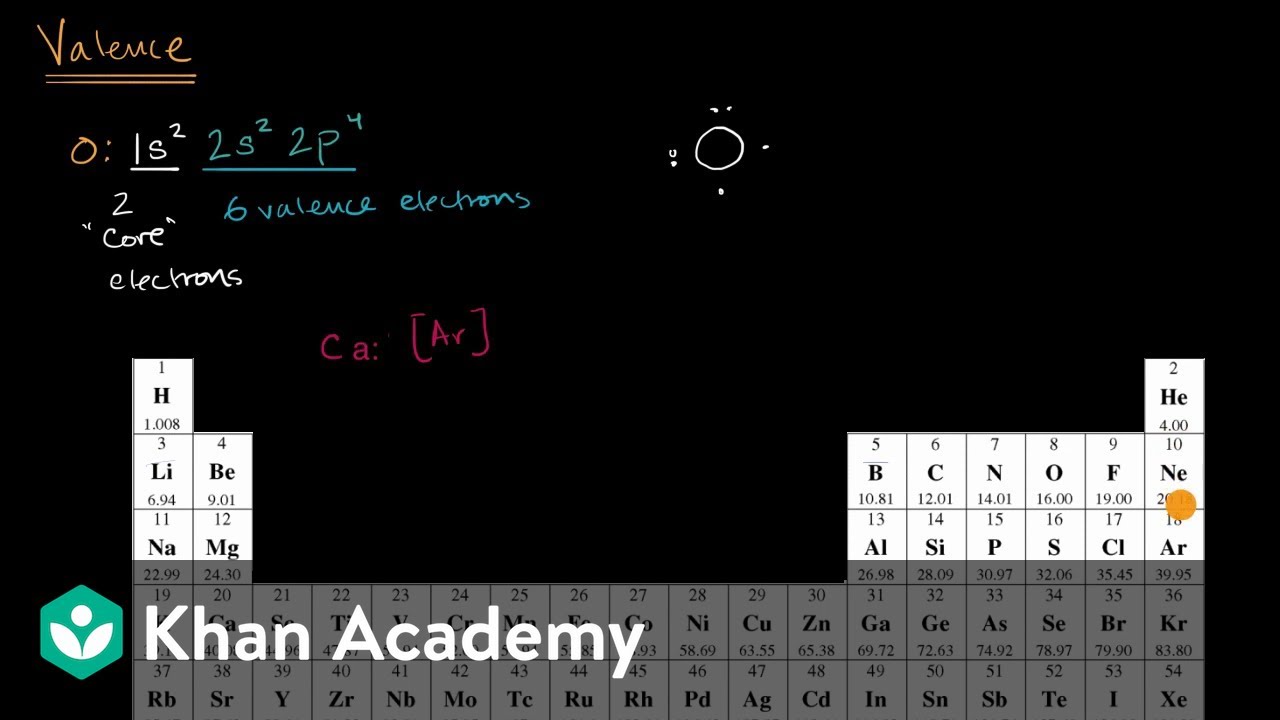
Lewis Diagram (Electron Dot) In most stable molecules or polyatomic ions, each atom tends to acquire a noble-gas structure by sharing electrons. This tendency is often referred to as the octet rule. One way to show the structure of an atom or a molecule is using dots to represent the outermost s-and p-electrons (the so-called valence electrons).
Valence electrons are the total number of electrons residing in the outermost shell or valence shell of the electronic configuration of an atom. These valence electrons act as the base structure for the Lewis dot structure. To form the initial structure, the total number of valence electrons, the initial structure can be formed.
Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom:
What is the Lewis dot structure of Na and Cl? The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet. In the case of the sodium cation, the filled shell is the outermost of the 'core' electron shells.
How does the Lewis dot diagram work? What is a covalent bond? Gilbert Lewis It uses a system of dots to show the valence electrons in a ch… A bond that forms when 2 or more atoms share electrons. 28 Terms lgray5214 Lewis Dot Diagrams Lone pair double bond Bonding pair A pair (2) of electrons not involved in bonding
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? When a metal atom combines with a nonmetal atom, the nonmetal atom will; Suatu atom dengan nomor atom 53 dan massa atom 127 mengandung; The pattern of _____ determines earth's precipitation pattern. The metalloid that has five valence electrons in the ...
Answer (1 of 2): You have got three atoms, first choose which one will be the central, and that is Oxygen. Now draw separately the atomic diagram of each atom so that you would know that how many valence electrons are in each one of them. Now you know that Oxygen has 6 electrons in its valence ...
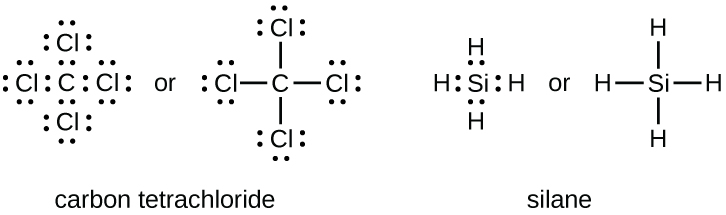
Carbon has four valence electrons and it needs four more electrons to complete eight electrons to its valence shell. So, in Lewis dot structure of the carbon atom is the central atom and two H and two F atoms are bonded to it with single covalent bonds.. The molecular geometry of the molecule is tetrahedral(in box form).
Which Lewis dot diagram shows an atom that needs two more electrons in its outermost shell? 1 See answer Advertisement Advertisement JubileeK is waiting for your help. Add your answer and earn points. KnSomers KnSomers D, fluorine needs two more electrons on the outermost shell. I hope this helps! Advertisement Advertisement
Therefore, put 2 electrons from the remaining valence electrons on nitrogen and complete its octet. Lewis dot structure for NCl3 As you see in the above structure, each atom (nitrogen and chlorine) has completed its octet as each of them has 8 electrons around them. So, this is the best and stable Nitrogen trichloride lewis structure we have made.
Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Lewis symbol: symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion. lone pair: two (a pair of) valence electrons that are not used to form a covalent bond.
Which Lewis dot diagram shows an atom that needs two more electrons in its outermost shell? Related Answer. Using electron-dot diagrams which show only the ...
A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (In table salt, this electron comes from the sodium atom.) \[\ce{e^{-} +Cl -> Cl^{-}}\] In this case, the ion has the same outermost shell as the original atom, but now that shell has eight ...
The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons.

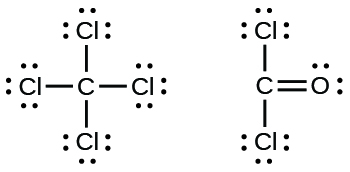





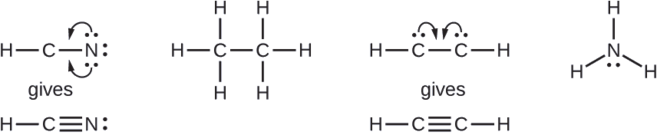
![Best Answer] Choose the write Lewis electron dot diagram for ...](https://us-static.z-dn.net/files/d88/47b81ffdeb97e849146b837bc52b2301.png)













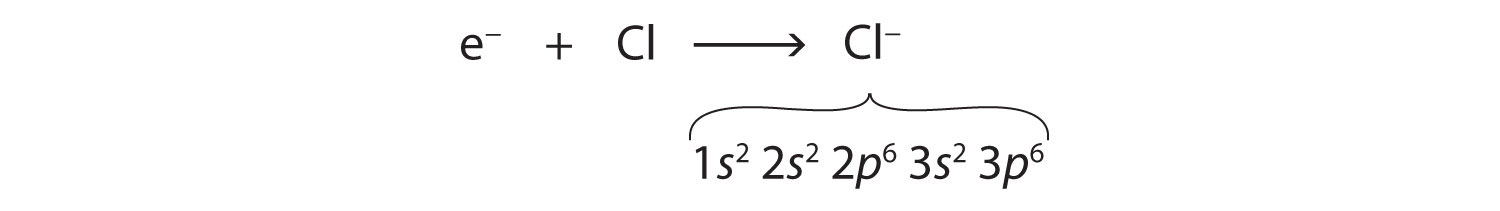
0 Response to "42 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell?"
Post a Comment