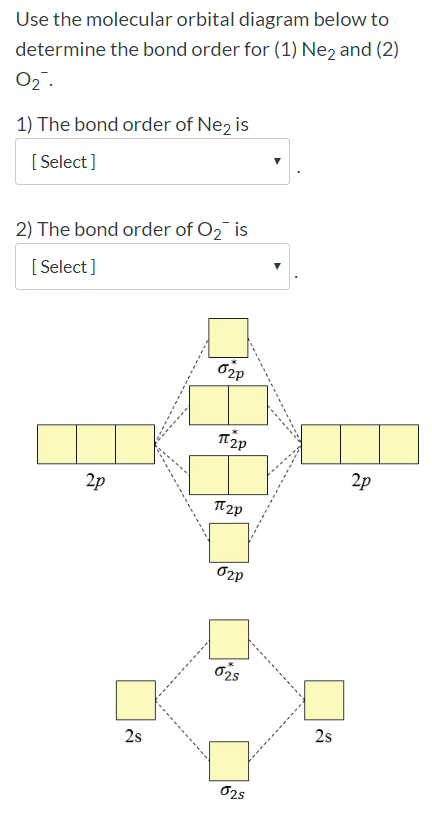
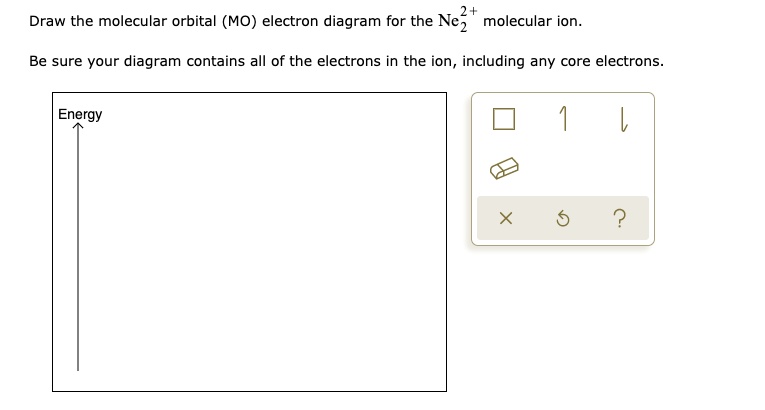
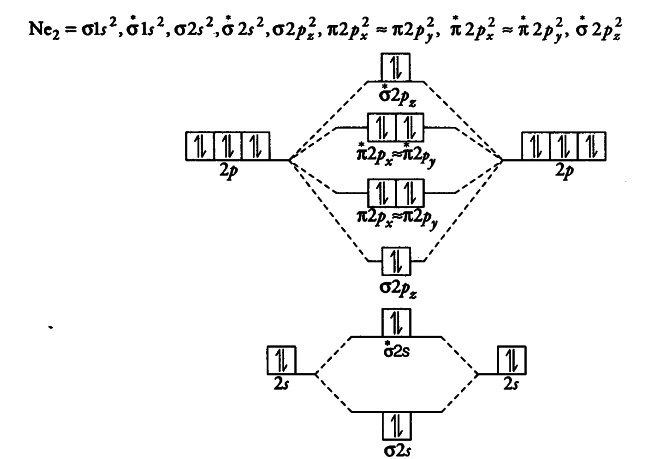
42 ne2 molecular orbital diagram
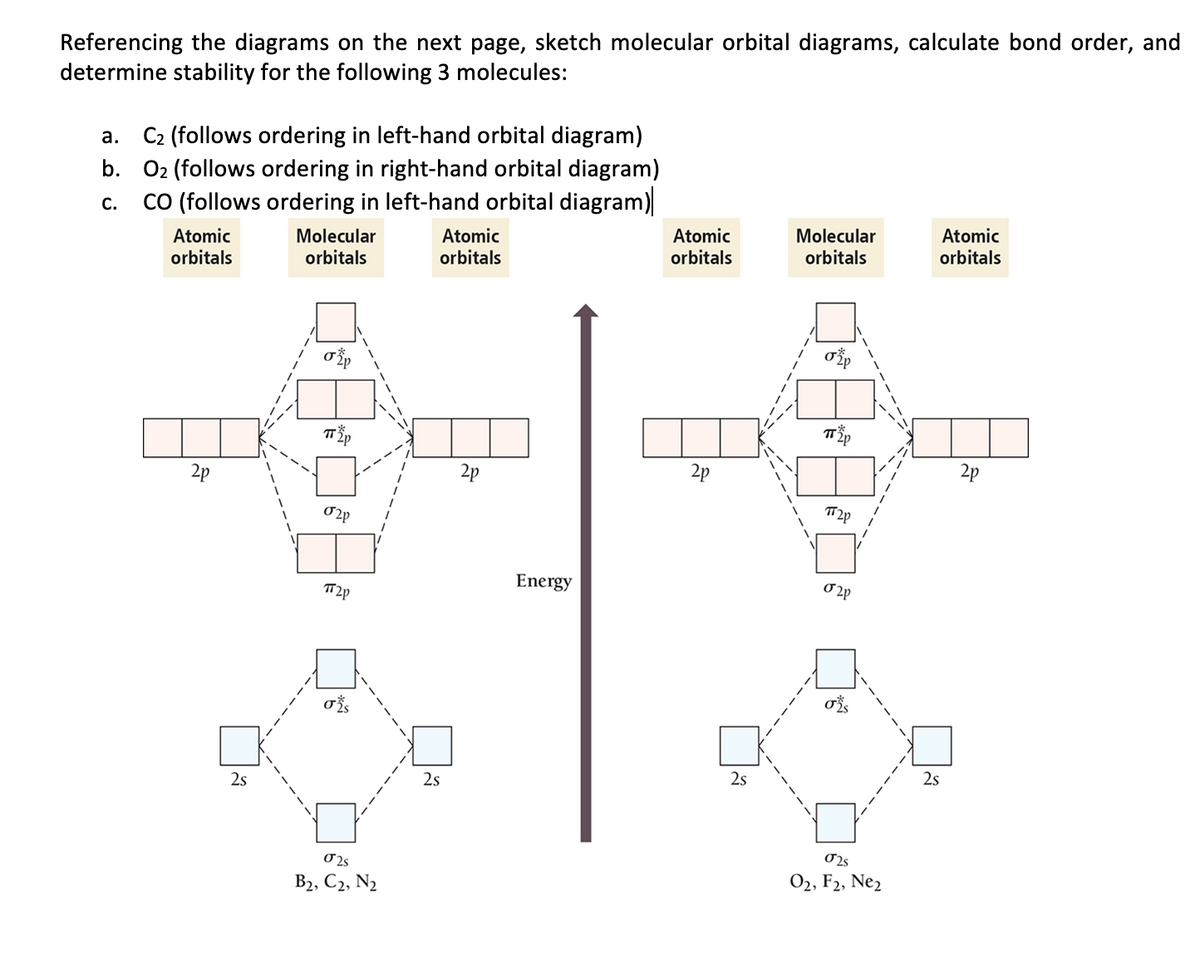
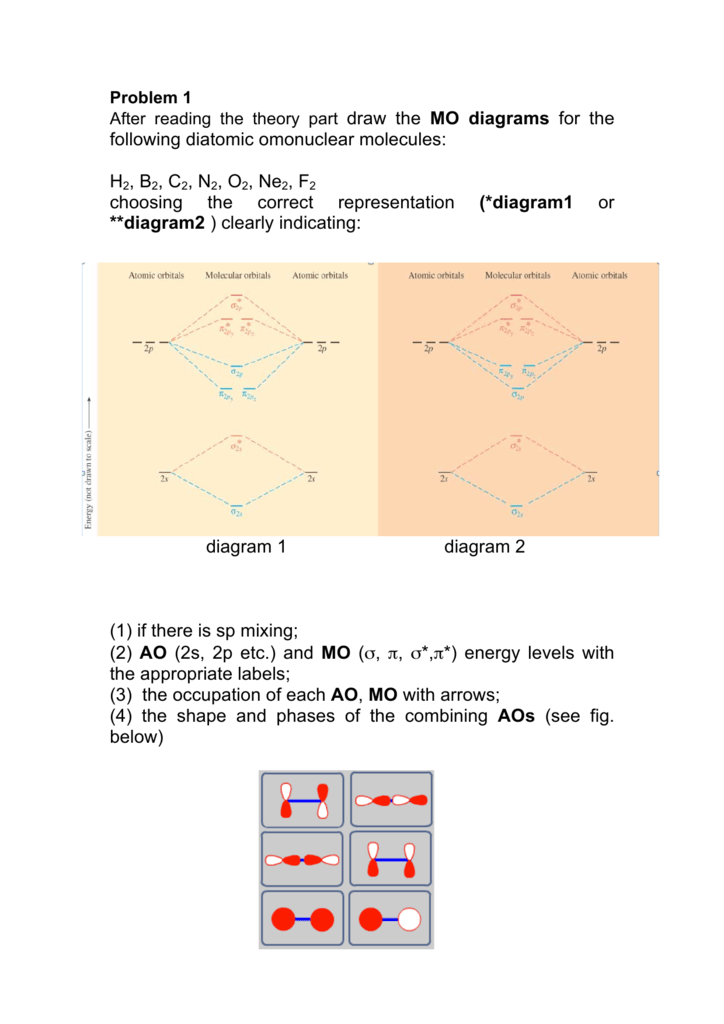
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Problem 1. After reading the theory part draw the MO diagrams for the following diatomic omonuclear molecules: H2, B2, C2, N2, O2, Ne2, F2.13 pages
MO Diagram of heteronuclear diatomic Molecules - Chemical Bonding & Molecular Structures - Inorganic. Molecular Orbital (MO) Diagram of Polyatomic molecules Beryllium dihydride (BeH2) and Water (H2O).

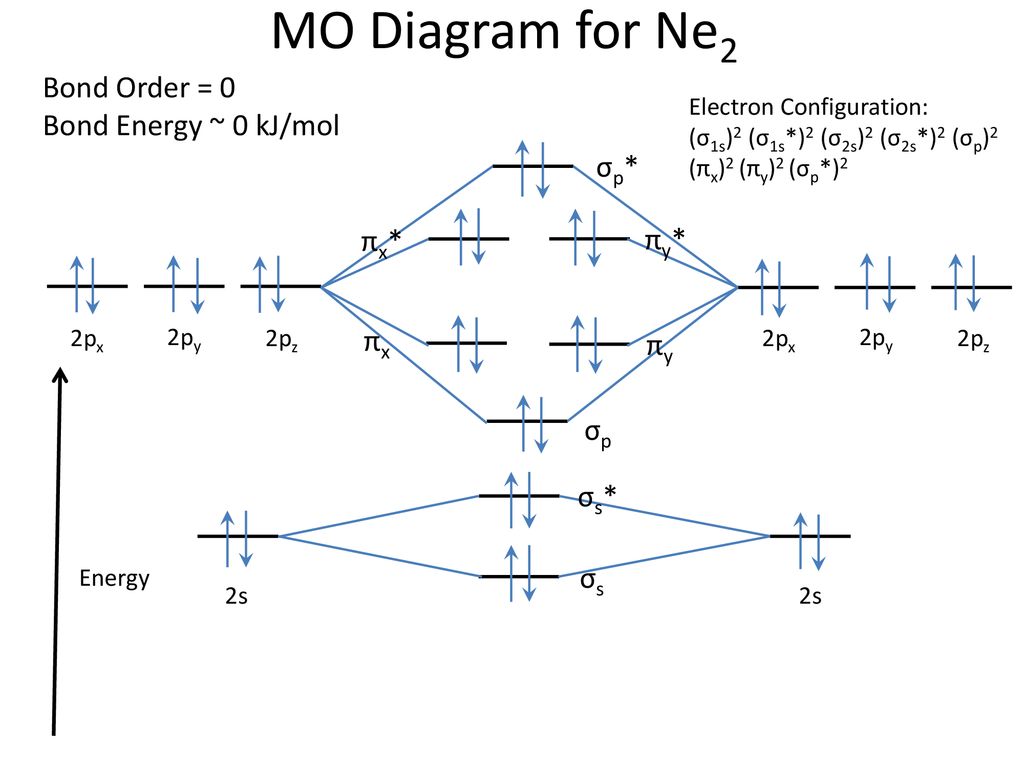
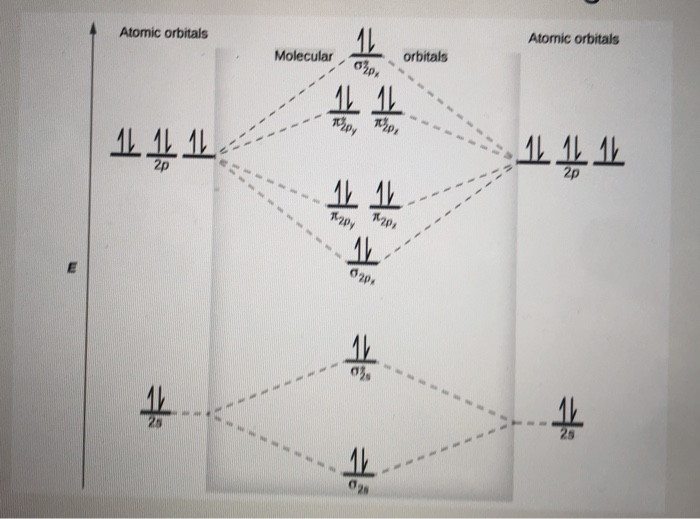
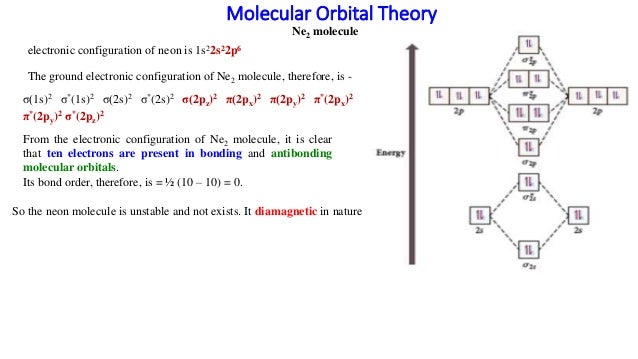
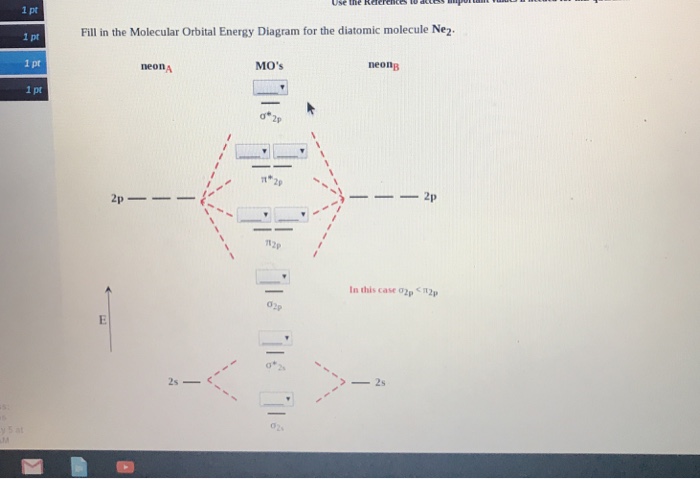
Ne2 molecular orbital diagram
A molecular orbital can hold two electrons, so both electrons in the H2 molecule are in the σ1s bonding orbital; the electron configuration is (σ1s)2. We represent this configuration by a molecular orbital energy diagram (Figure 5.50) in which a single upward arrow indicates one electron in an... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the However, we can predict that the Be2 molecule and the Ne2 molecule would not be stable. We can see this by a consideration of the molecular electron...
Ne2 molecular orbital diagram. 3.2.2019 · sorry about that not being a molecular orbital diagram i saw orbital and immediately thought electron configuration to save confusion, could. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Two superpositions of these two orbitals can be formed, one by summing the orbitals and the other by taking their difference. In the former, the amplitudes of Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the... Biblioteca en línea. Materiales de aprendizaje gratuitos. 1000 Solved Problems in Classical Physics Ahmad A. Kamal 1000 Solved Problems in Classical Physics An Exercise Book 123 Dr. Ahmad A. Kamal Silversprings Lane 425 75094 …
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. Molecular orbitals are the energy states of a molecule in which the electrons of the molecule are filled just as atomic orbitals are the energy states of an atom in which No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule... Draw out the molecular orbital diagram for Ne2, starting with the 2s atomic orbitals and label each molecular orbital with the appropriate notation as done ...4 answers · Top answer: So here we're looking at the molecular orbital theory to describe bonding. So the first example, ... The Molecular Orbital Theory is a chemical bonding theory developed at the turn of the twentieth century by F. R. Hund and R. S. Mulliken to explain the structure The valence-bond theory failed to adequately explain how certain molecules, such as resonance-stabilized molecules, contain two or...
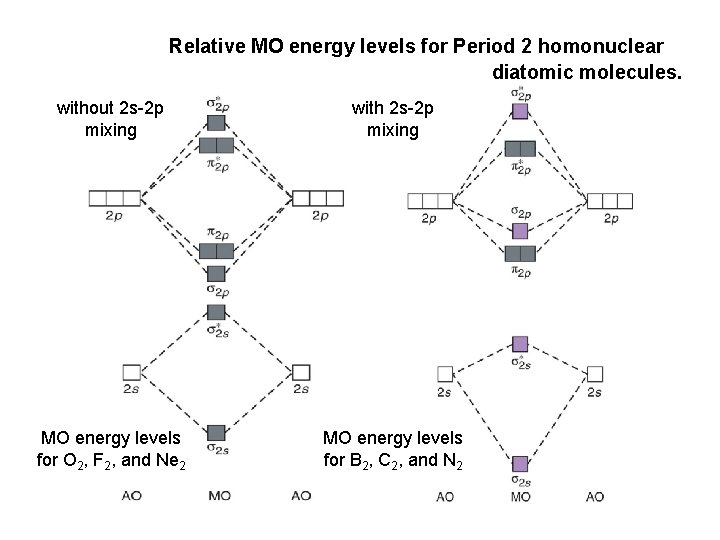
Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in Diagrams such as these are used to describe the bonding in a molecule in MO terms. Electrons occupy MOs according to the same rules developed for atomic orbitals; they... Academia.edu is a platform for academics to share research papers. A molecular orbital diagram, or MO diagram for short, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.[1][2][3] A... 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently lower in energy than the valence Does your MO diagram agree with this expectation? Determine the primary MOs that determine the bond order.
Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the One of the molecular orbitals in this molecule is constructed by adding the mathematical functions The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
Question : molecular orbital diagram ne2. This problem has been solved! See the answer done loading. molecular orbital diagram ne2.
Free NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure solved by expert teachers from latest edition books and as per NCERT (CBSE) guidelines.Class 11 Chemistry Chemical Bonding and Molecular Structure NCERT Solutions and Extra Questions with Solutions to help you to revise complete Syllabus and Score More marks.
Material Science And Engineering V Raghavan.pdf [qwy1v9k79ywm]. Materials Scienceand Engineering A First CourseFIFTH EDITION V. Raghavan 1 2H He1.008 THE PERIODIC TABLE 4.0031s1 1s2 3 ...
Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. Have you ever thought about how sigma and pi bonds are formed? What is the difference between diamagnetic and paramagnetic behaviour?
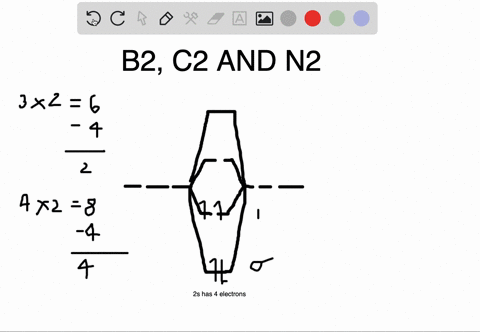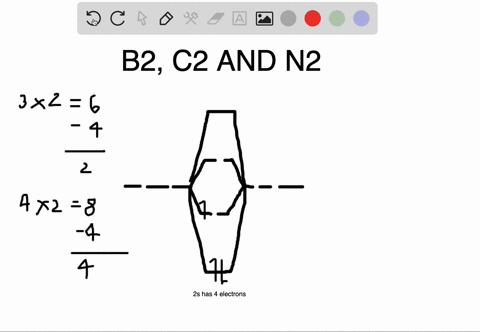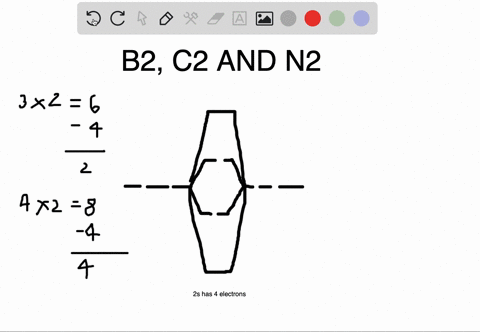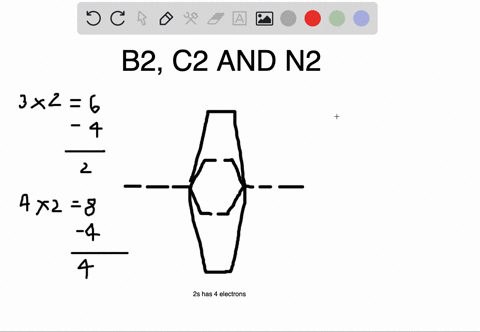
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. The video below describes how to generate molecular orbital diagrams for B₂ and other diatomic molecules from Row 2 elements of the...
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
• Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
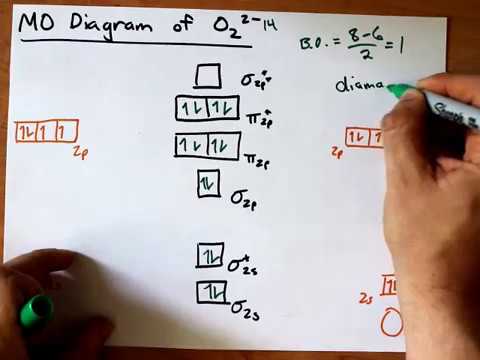
What is Molecular Orbital Theory. With the help of energy levels homonuclear diatomic orbitals, arrange the following species in increasing order of stability O22− ,O2 −,O2 ,O2 +. > Draw the molecular orbital diagram of dioxygen and calculate bond order.
Academia.edu is a platform for academics to share research papers.
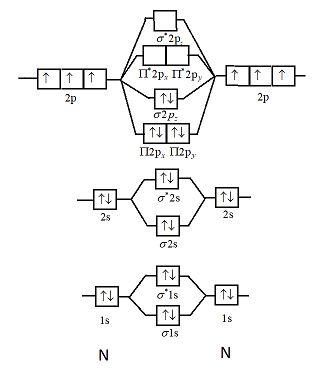
Bond Order. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Li2, Be2, B2, C2, N2, O2, F2, and Ne2.
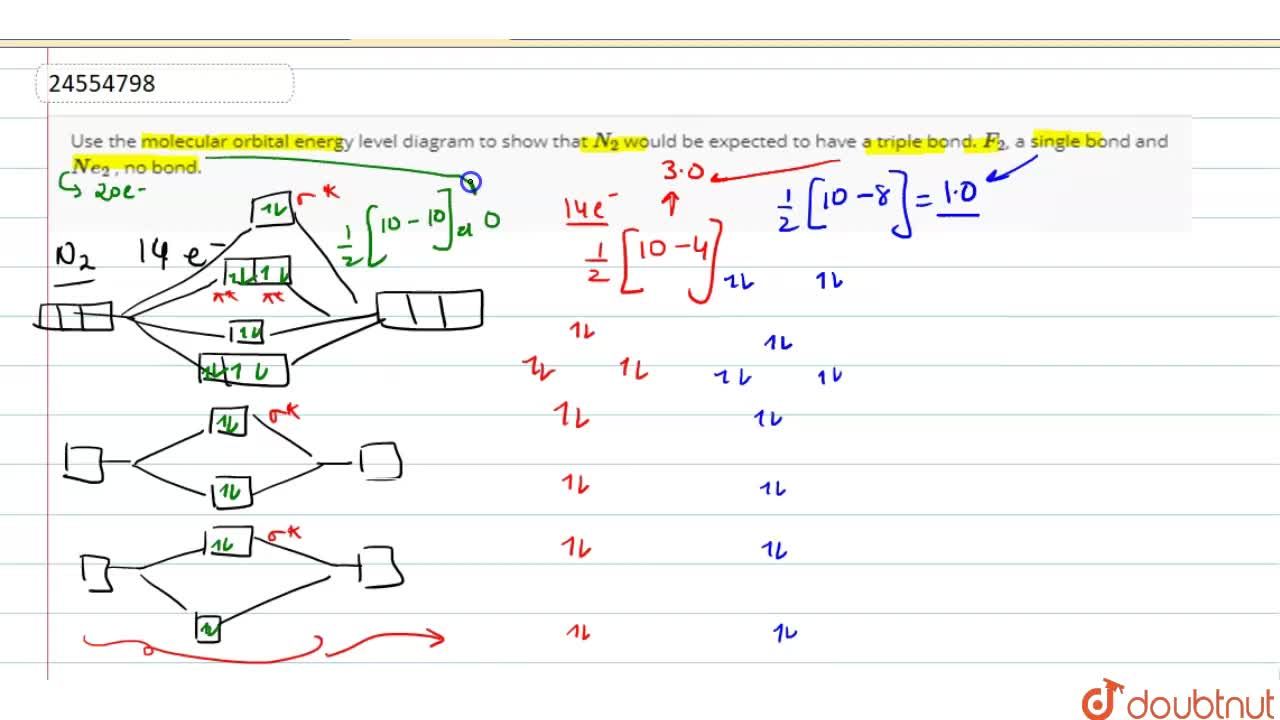
Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2, a single bond and Ne2, no bond. · Solution.1 answer · Top answer: Formation of N2 molecule: Electronic Configuration, σ 1s^2<σ *1s^2<σ 2s^2<σ *2s^2<[pi 2px^2 = pi 2px^2]<<σ 2pz^2 Bond order = (Nb - Na)/2 = (10 - ...
26 Jan 2020 — With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). chemical bonding ...1 answer · Top answer: Ne2 (20) = σ1s2 σ*1s2, σ2s2 σ*2s2, σpx2 π 2py2 2π* 2py2π*2py2 2pz2 σ* 2px2 B.O = 1/2 (10 - 10) = 0 Ne2 cannot exist because its bond order is zero.
Molecular orbital diagram of. Explanation: Neon atom has 10 electrons and its electronic configuration is . When molecule is considered, it has two neon atoms and thus is composed of 20 electrons. The electronic configuration and bond order of molecule is as follows
8 Molecular Orbital Theory Each line in the diagram represents an orbital. The molecular orbital volume encompasses the whole molecule. 17 Molecular Orbital Diagram (CH4) So far, we have only look at molecules with two atoms. MO diagrams can also be used for larger molecules.
Polyatomic Molecular Orbital Theory. Transformational properties of atomic orbitals. B2 10 1 2. C2 12 2 0 N2 14 3 0 O2 16 2 2 F2 18 1 0 Ne2 20 0 0. The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure.
The molecule Ne2 is predicted to be unbound (bond order of 0), and has indeed never been observed. For the elements Li to N, the molecular orbital From this diagram, we can obtain the ground state electronic configurations for all the diatomic molecules of the elements from Li to N. This leads to...
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. However, we can predict that the Be2 molecule and the Ne2 molecule would not be stable. We can see this by a consideration of the molecular electron configurations (Table 3).
This second orbital is therefore called an antibonding orbital. Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable.
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the However, we can predict that the Be2 molecule and the Ne2 molecule would not be stable. We can see this by a consideration of the molecular electron...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
A molecular orbital can hold two electrons, so both electrons in the H2 molecule are in the σ1s bonding orbital; the electron configuration is (σ1s)2. We represent this configuration by a molecular orbital energy diagram (Figure 5.50) in which a single upward arrow indicates one electron in an...

















0 Response to "42 ne2 molecular orbital diagram"
Post a Comment