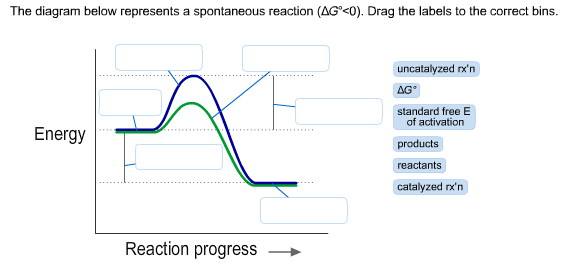
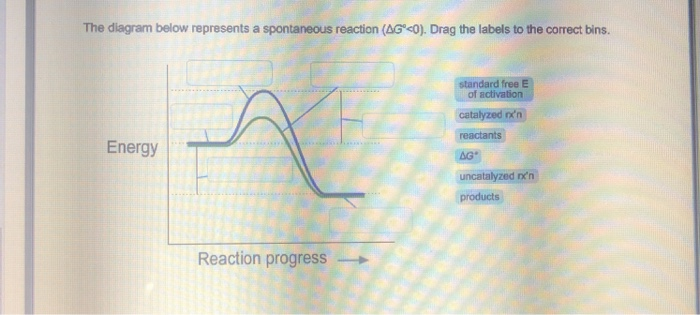
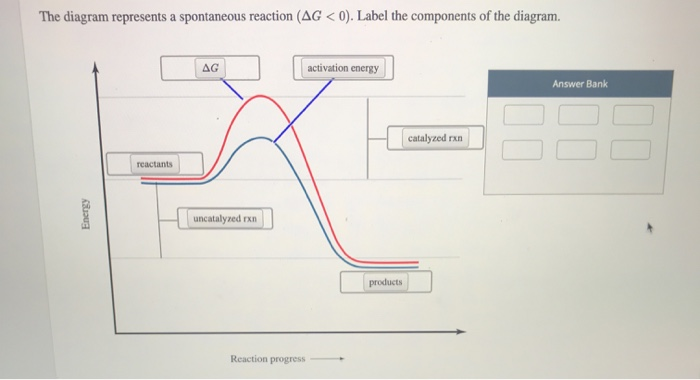
42 the diagram below represents a spontaneous reaction (δg
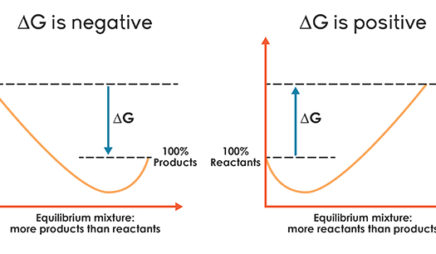
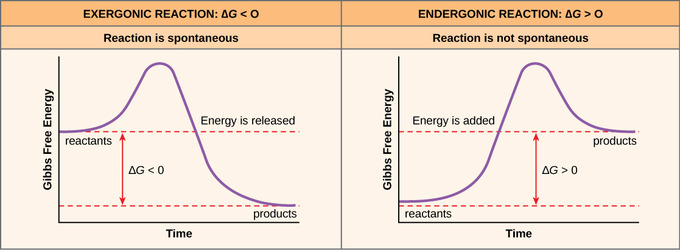
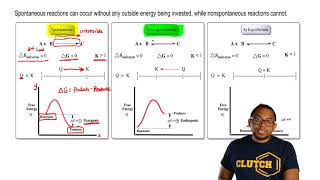
For spontaneous reactions, ΔG must be less than zero and for... Classify the possible combinations of signs for a reaction's ∆H and ∆S values by the resulting spontaneity. For checking the spontaneity of a reaction, we have to check the sign of ΔG using the below formula. Gibbs Free Energy and Spontaneous Reactions This reaction is, in fact, an exergonic reaction, which is a fancy way of saying that the reaction Also, the graph of the exergonic/spontaneous reaction above reveals that the free energy of the products Of course, the equation for free energy: ΔG=ΔH−TΔS\, reminds us that spontaneity is about three...
saplinglearning.com/ibis/css/Accessibility.php?text=The diagram... If you can't find your institution, please check your spelling and do not use abbreviations. If your institution is not listed, please visit our Digital Product Support Community. The diagram below represents a spontaneous reaction (ΔG.
The diagram below represents a spontaneous reaction (δg
41 the diagram below represents a spontaneous reaction (δg The Diagram Represents A Spontaneous Reaction Use The. 6 hours ago Although we can write any chemical reaction on paper not all chemical reactions will Hence reactions are spontaneous only when ΔG, the change in free energy, is negative. The overall situation is summed up in the Gibbs... The diagram below represents a spontaneous ... - HomeworkLib In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous reaction.1 answer · 0 votes: Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous ... Gibbs Free Energy | Chemistry [Master] spontaneous change: A spontaneous process is the time-evolution of a system in which it releases If the reaction is sufficiently exothermic it can force ΔG to be negative only at temperatures below If the reaction starts at a composition to the right of point 3 on the diagram, the composition will tend...
The diagram below represents a spontaneous reaction (δg. The diagram below represents a spontaneous reaction (deltaG The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? endothermic Draw two reaction energy diagrams that illustrate the type of results expected for the reaction below, one diagram for the reaction in the... Difference Between Spontaneous and Nonspontaneous Reactions... Spontaneous reactions refer to the chemical reactions that occur without being driven by an outside force. The two driving forces of a chemical reaction are enthalpy and entropy. Enthalpy is a thermodynamic property of a system that is the sum of the internal energy added to the product of the... The Diagram Below Represents A Spontaneous Reaction δg In A Dataflow Diagram Dfd Aan Represent Data Stores. Honda Fit Body Parts Diagram. Johnson Outboard Starter Solenoid Wiring Diagram. In The Diagram Which One Represents Carrier Mediat... Free Body Diagram Of A Pulley. Craftsman Lt2000 Deck Belt Diagram. Samacheer Kalvi 12th Chemistry Guide Chapter 1 Metallurgy 18-12-2020 · For a spontaneous reaction, the change in free energy ∆G should be negative. Thermodynamically the reduction of metal oxide [equation (1)] with a given reducing agent [equation 2,3, or 4] can occur if the free energy change for the coupled reaction [equation 1&2, 1&3 or 1&4] is negative.
Gibbs Free Energy and Spontaneity Chemistry Tutorial ΔG represents the change in Gibbs free energy for a chemical system at constant temperature The table below summarises the values for the change in Gibbs free energy for spontaneous reactions Reactions proceed spontaneously to minimise enthalpy (by releasing heat) and maximise entropy... 11.5: Spontaneous Reactions and Free Energy - Chemistry LibreTexts A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring. A roaring bonfire (see figure below) is an example of a spontaneous reaction. Graphene Oxide Causes Loss of Taste, Smell... - katrinah.com For example, 100 patients with saturation levels below 50% practically dead —bluntly speaking—, with bilateral pneumonias, within an hour of intravenous glutathione or N-acetylcysteine administration they made it. They were taken off ventilators and everything. Oxidative Phosphorylation | ΔGo= -61.9 kJ (spontaneous) Separate chemical reactions may be added together to form a net reaction. The free-energy change (ΔG) for the net This is a schematic diagram showing the membranes of the mitochondrion. The purple shapes on the inner membrane represent proteins, which are described in the section below.
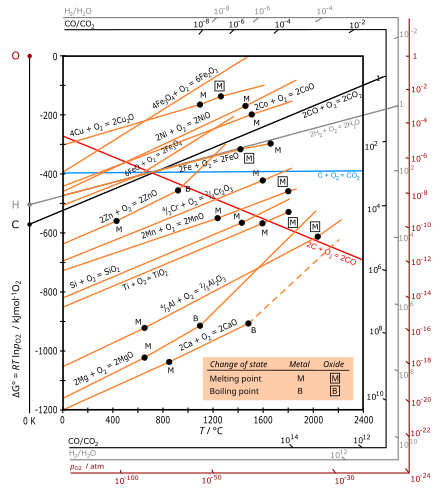
If ΔG > 0, then the forward reaction is spontaneous. | Brainscape When ΔG > 0, the reverse reaction is spontaneous. Look at the diagram for the fusion of water (from liquid to solid). Which statement about Point B is not correct? A reaction that is exothermic and causes a decrease in the entropy of the system cannot be a spontaneous reaction. Ellingham Diagrams (all content) | Partial pressure of reacting gas The diagram is essentially a graph representing the thermodynamic driving force for a particular reaction to ΔG° for a reaction is hence a very useful value to know. The Ellingham diagram. Consider the two oxidation reactions below, whose lines on the Ellingham diagram cross each other Chapter 1: The Foundations of Biochemistry – Chemistry if ΔG = 0, the reaction is at equilibrium and no further change occurs in the concentration of reactants and products. if ΔG > 0, the reaction goes toward reactants A and B. We can not measure easily the actual free energy G of reactants or products, but we can measure ΔG readily. These points are illustrated in the graph below of ΔG vs ... UCF CHM 2046 DIXON EXAM 4 REVIEW PRACTICE... | Course Hero Ucf - chemistry fundamentals 2 - prof. DIXON UCF CHM 2046 DIXON EXAM 4 REVIEW*PRACTICE:The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe blanksbelowPRACTICE:What is the value of K for this aqueous reaction at 298 K?
Gibbs free energy - Wikipedia In thermodynamics, the Gibbs free energy (or Gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.The Gibbs free energy (=, measured in joules in SI) is the maximum amount of non-expansion work that can be extracted …
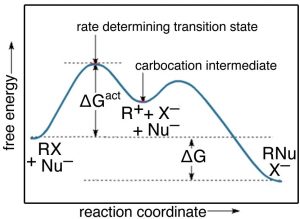
CONNECT CHAPTER 10 METABOLISM REVIEW Flashcards | Quizlet Non-spontaneous. +ΔG. What state exists when the rates of chemical reactions in both directions Which of the labels in the image represents a region of the reaction that is most susceptible to In this common diagram of enzyme activity, which of the labels in the image represents the total free...
Electrochemistry Basics - Chemistry LibreTexts 07-08-2021 · For a spontaneous reaction, E cell is positive and ΔG (Gibbs free energy, used to determine if a reaction occurs spontaneously) is negative. Thus, when ΔG is negative the reaction is spontaneous. Merging electrochemistry with thermodynamics gives this formula: \[\Delta G = -n F E_{cell} \]
(PDF) ESSENTIAL CELL BIOLOGY ESSENTIAL CELL ... - Academia.edu Academia.edu is a platform for academics to share research papers.
The Diagram Represents A Spontaneous Reaction Use The... Use the diagram to answer the questions below. T c the equivalence point occurs when equal moles of substances react. Kj c what is the enthalpy change h for the forward reaction. 2789184 home questions sciencemath chemistry chemistry others the diagram represents a spontaneous reaction.
Gibbs free energy | ΔG changes with temperature Because you are calculating ΔG, your answer will be in kJ mol-1. You must remember to change the entropy change value into kJ before you start, otherwise you will get the calculation completely wrong. On an earlier page in this section, we calculated the entropy change for the reaction.
Equilibrium and Advanced Thermodynamics... - Annenberg Learner Δg = δh - tδs. For a spontaneous reaction, ΔG will be negative; for a nonspontaneous reaction, ΔG will be positive. Osmosis and Entropy The natural progression toward greater entropy sometimes produces surprising results. The diagram below shows a U-shaped tube divided into two halves by a...
Prelim Chemistry : Explaining Spontaneous Reactions - Art Of Smart... Struggling with explaining spontaneous reactions in Prelim Chemistry? Watch these videos to learn more and ace your Prelim Chemistry Exam! Solve problems using standard references and ΔG∘=ΔH∘−TΔS∘ (Gibbs free energy formula) to classify Determining if a Reaction is Spontaneous.
The diagram below represents a spontaneous reaction (δg In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous reaction. The blue curve represents the uncatalyzed reaction and the green curve represents the catalyzed reaction. Drag the labels in the diagram as follows
Spontaneous process - Wikipedia A spontaneous reaction is a chemical reaction which is a spontaneous process under the conditions of interest. where the sign of ΔG depends on the signs of the changes in enthalpy (ΔH) and entropy (ΔS). The sign of ΔG will change from positive to negative (or vice versa) at the temperature given by...
The Diagram Below Represents A Spontaneous Reaction δg Note The Diagram Below Which Shows A Circuit Creat... Refer To The Diagram For A Purely Competitive Prod... Ford Explorer Door Latch Diagram. Complete The Diagram Below Using The Following Steps. Chevy S10 Stereo Wiring Diagram. Onan Rv Generator Wiring Diagram.
Chapter 9 - Reaction Energetics The enthalpy of reaction, ΔH, is the heat absorbed by a reaction when it is carried out at constant temperature and pressure. Most enthalpies of reaction used in this course are standard enthalpies. A thermochemical equation is a balanced chemical equation that includes a thermodynamic property...
(PDF) Hopkins W.,Huner N.-Introduction to plant physiology ... Hopkins W.,Huner N.-Introduction to plant physiology-2008.pdf
C:\exams\Supplementary\Answer Keys\Chemistry 3202 ... Which represents the activation energy for the forward reaction in the ... According to the collision theory, why does the reaction below occur in more than ...16 pages
16.4: The Nernst Equation - Chemistry LibreTexts 13-04-2021 · which represents the transport of cupric ion from a region of higher concentration to one of lower concentration. The driving force for this process is the free energy change ΔG associated with the concentration gradient (C 2 – C 1), sometimes known as the free energy of dilution: \[ΔG_{dilution} = RT \ln(C_2 – C_1)\]
Solved The diagram below represents a spontaneous reaction Transcribed image text: The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n.
For spontaneous reaction, ΔG is For a spontaneous reaction, the sign on ΔG must be negative. Gibbs free energy relates enthalpy, entropy and temperature. A spontaneous reaction will always occur when ΔH is negative and ΔS is positive.
The Diagram Below Represents A Spontaneous Reaction δg The arrows in the diagram could represent the release of 1 atp from a chloroplast carrying out photosynthesis 2 oxygen from a mitochondrion...
Nelson Biology 12.pdf [30j71j2z320w] - doku.pub As a result, the second reaction (the reverse reaction) will occur more frequently, and the H1 ions will be absorbed as carbonic acid molecules form. Conversely, if there are very few H1 ions in solution, they will not collide as frequently with the bicarbonate ions, and the reverse reaction will occur at a slower rate than the forward reaction (the first equation above).
Transition metal It reacts with aqueous iron(II) ions as shown in the equation below. The enthalpy change for this reaction is approximately zero. Two repeating units in the polymer chain are shown. Each iron ion is also bonded to two water molecules. These are not shown in the diagram. (a) Name the type of bond...
PDF Choose the substance with the highest viscosity. 4. Refer to the diagram below. Begin at point b and lower the pressure at constant temperature. A. The reaction will shift to the left in the direction of the reactants. B. No effect will be observed. A. ΔG is proportional to the negative of ΔSuniverse. B. ΔG < 0 represents a spontaneous process.
exercises - chemical thermodynamics - chemistry the central science 19.8 The accompanying diagram shows how ΔG for a hypothetical reaction changes as temperature changes. (a) At what temperature is on the right side of the diagram, represent? 19.12 Which of the following processes are spontaneous: (a) the melting of ice cubes at -10 °C and 1 atm pressure...
Gibbs Free Energy | Chemistry [Master] spontaneous change: A spontaneous process is the time-evolution of a system in which it releases If the reaction is sufficiently exothermic it can force ΔG to be negative only at temperatures below If the reaction starts at a composition to the right of point 3 on the diagram, the composition will tend...
The diagram below represents a spontaneous ... - HomeworkLib In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous reaction.1 answer · 0 votes: Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous ...
41 the diagram below represents a spontaneous reaction (δg The Diagram Represents A Spontaneous Reaction Use The. 6 hours ago Although we can write any chemical reaction on paper not all chemical reactions will Hence reactions are spontaneous only when ΔG, the change in free energy, is negative. The overall situation is summed up in the Gibbs...

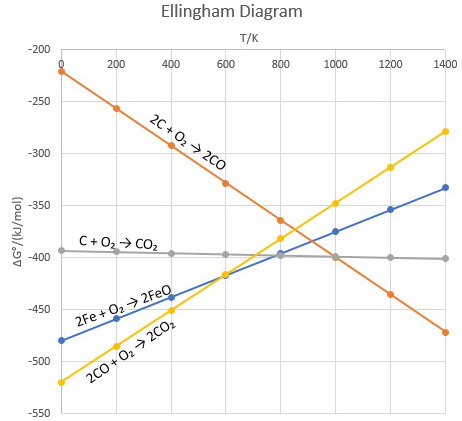



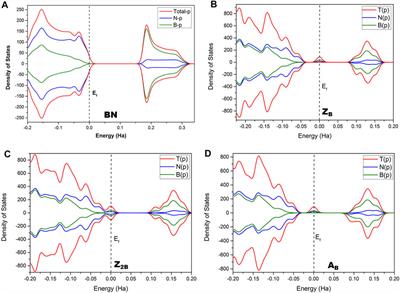




:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)




/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)

0 Response to "42 the diagram below represents a spontaneous reaction (δg"
Post a Comment