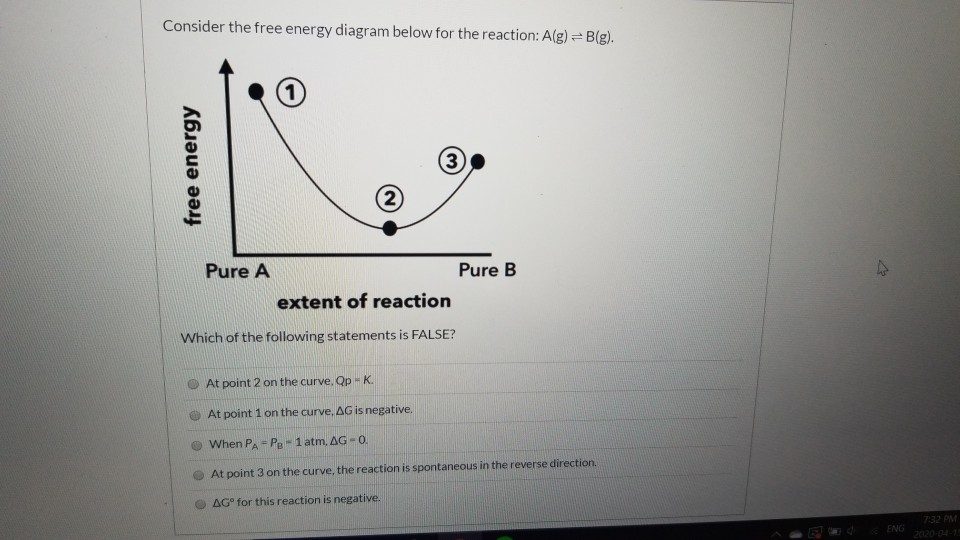
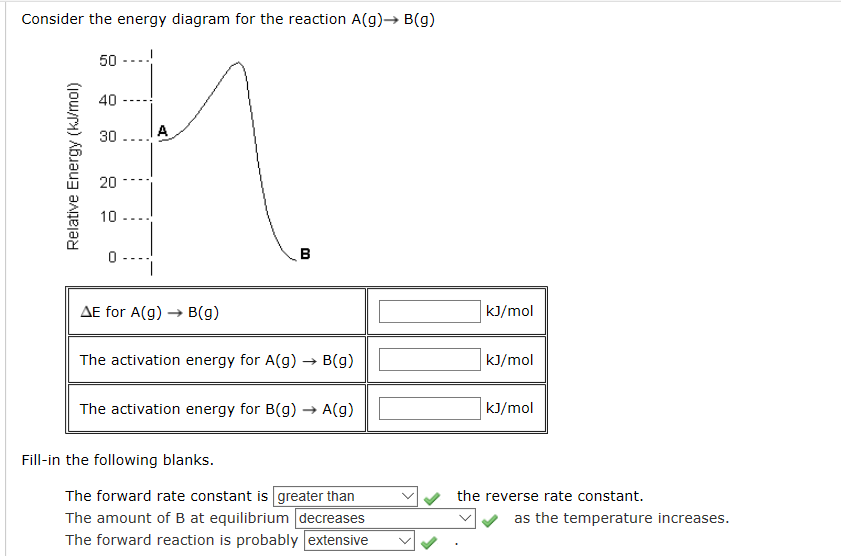
42 Consider The Energy Diagram For The Reaction A(g) B(g)
16.4 Free Energy - Chemistry Free energy is a state function, so its value depends only on the conditions of the initial and final states of the system that have undergone some change. Recall that Q is the numerical value of the mass action expression for the system, and that you may use its value to identify the direction in which a... Consider the energy diagram below. A graph of reaction progression... A graph of reaction progression on the horizontal axis versus energy on the vertical axis. A line starts flat low on the vertical axis, the flat area is labeled A. It rises sharply to peak, labeled C, then falls a short distance before levelling off, labeled D. A dotted Which statement best describes the diagram?
Solved Consider the following diagram of free energy (G ... Question: Consider the following diagram of free energy (G) versus fraction of A reacted in terms of moles for the reaction 2A(g) →B(g). G 0 0.33 0.67 Fraction of A reacted 1.00 Before any A has reacted, PA = 3.0 atm and PB = 0. Determine the sign of AGº and the value of K, for this reaction.

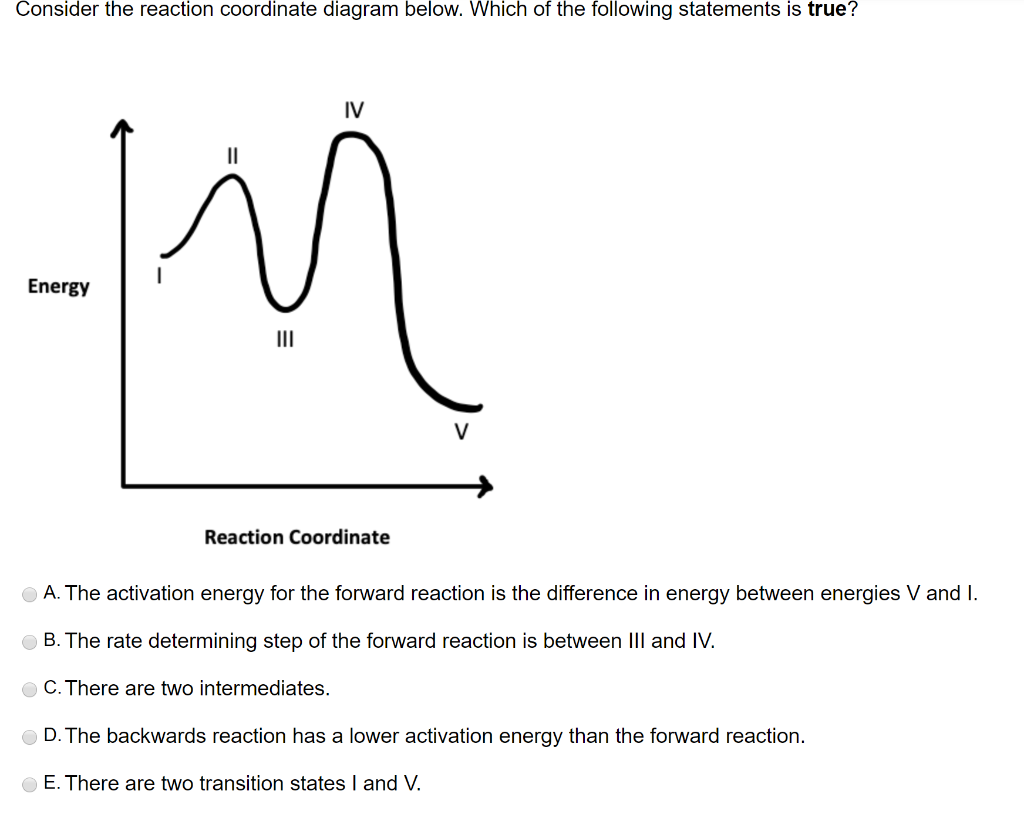
Consider the energy diagram for the reaction a(g) b(g)
At a certain temperature, nitrogen and hydrogen react | Chegg.com Transcribed image text: At a certain temperature, nitrogen and hydrogen react to form ammonia: N2(g) + 3H2(g)=2 NH3(g) When initial amounts of N2, H2, and NH3 are OK,>Q OK,«Q OK,-Q OK -Q-O OK, O Consider the free energy diagram below for the reaction: A(g) = B(g). free energy Pure A Pure... Chapter 13 Q's Flashcards - Quizlet Consider the following energy diagram and determine which of the following statements is true. At equilibrium, we would expect the concentration of the products to get greater than the concentration of the reactants because the activation energy is lower for the forward reaction. The diagram for the free energy of the rea... | Clutch Prep Problem: The diagram for the free energy of the reactionA (g) + B (g) ⇌ AB (g)The reaction progress starts on the left with pure reactants, A and B, each at 1 atm and moves to pure product, AB, also at 1 atm on the right. Select the true statements.a. The difference between the top left of the curve and the minimum of the curve corresponds to ...
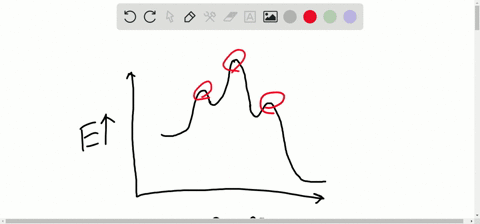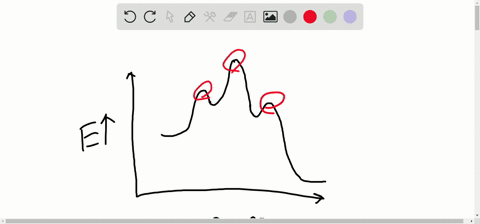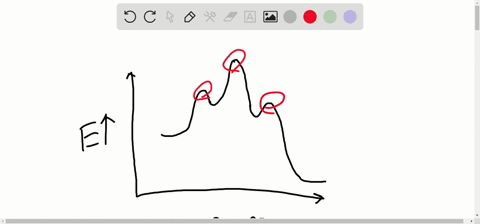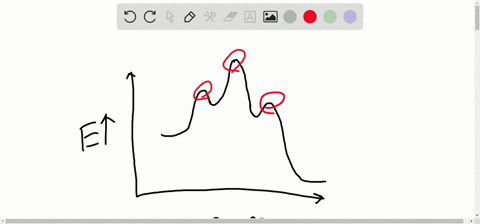
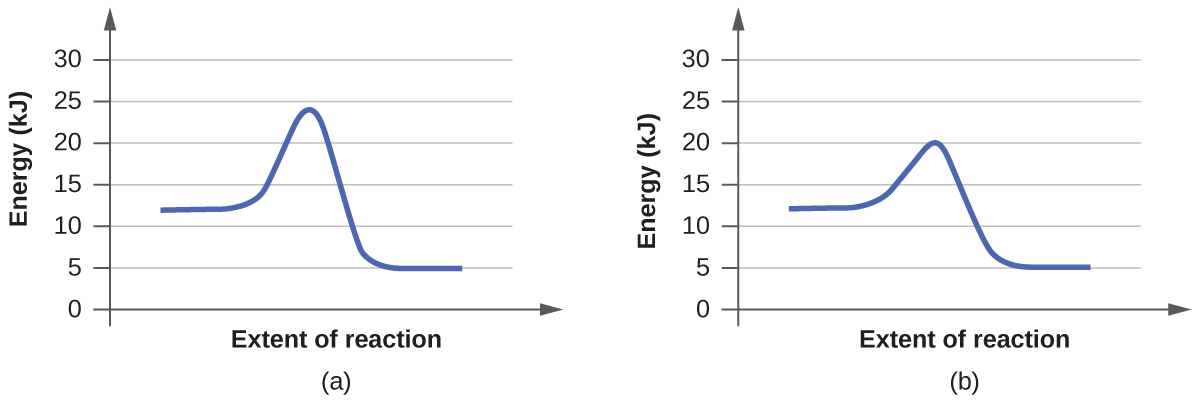
Consider the energy diagram for the reaction a(g) b(g). Chapter 6 Chem 51a f14 | PDF | Catalysis | Chemical Equilibrium Consider the following reaction: mA. B. Energy Diagrams An energy diagram is a schematic representation of the energy changes that take place as reactants are converted to products. Look at the following energy diagram for the a concerted reaction between molecule AB with C: to form A... Energy profile (chemistry) - Wikipedia For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products. The reaction, 2A(g) + B(g) 3C(g) + D(g) is begun with the... > Consider the reaction 2A⇌B. The equilibrium constant is 1×102. > For an equilibrium reaction 2A⇌P , if the reaction is started with one mole of 'A' and x is degree of dissociation, equilibrium concentrations of A and P are [1−x]2 > Important Diagrams. > Mindmap. > Problem solving tips. How to know a chemical equation is exothermic or endothermic... - Quora If the energy diagram is not available, the sign of the energy value signifying the kind of the chemical equation!!! This extra energy was needed for the reaction to occur, making it a reactant (thus, the reaction is endothermic). Consider the reaction
Exam #2: Concept Review Flashcards - Quizlet The diagram represents an equilibrium mixture for a reaction of the type B(g) + C(g) <---> BC(g). Determine the magnitude of K for the reaction. Assume each molecule or atom represents 1 mole of the species and that the volume of the container is 1 L. PDF Review for Midterm A reaction that releases Gibbs free energy is known as "exergonic". Proteins are polymers made of amino acids. Consider the formation of the dipeptide (a two amino-acid unit) alanylglycine from alanine and glycine, which is the first step in the synthesis of a protein molecule Energy Band Diagram - an overview | ScienceDirect Topics The energy band diagram of a quantum well is shown in Fig. Having presented an energy band diagram interpretation of our current understanding of the electronic properties of the MIV micro-emission regime, we are now able to describe how electrons are emitted from such a micro-regime. How to draw the potential energy diagram for this reaction? | Socratic 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic , measures the sum of its internal and potential energy. The reaction here see a decrease in chemical potential energy and shall, therefore, has a...
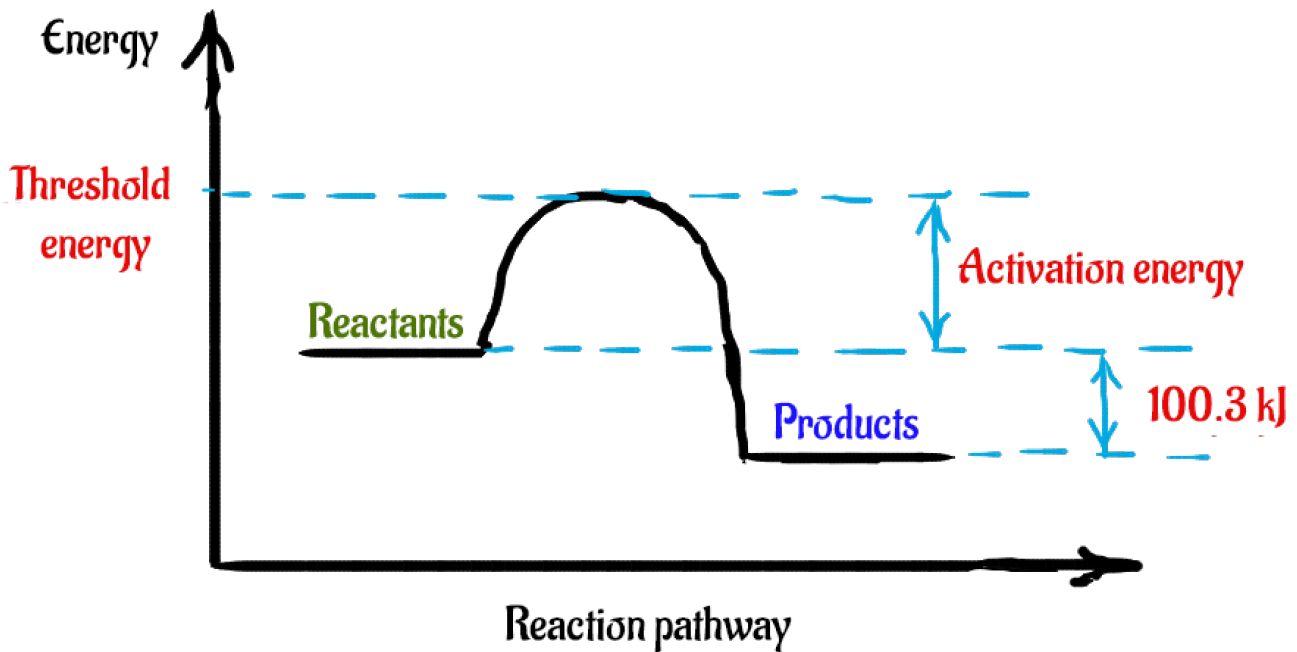
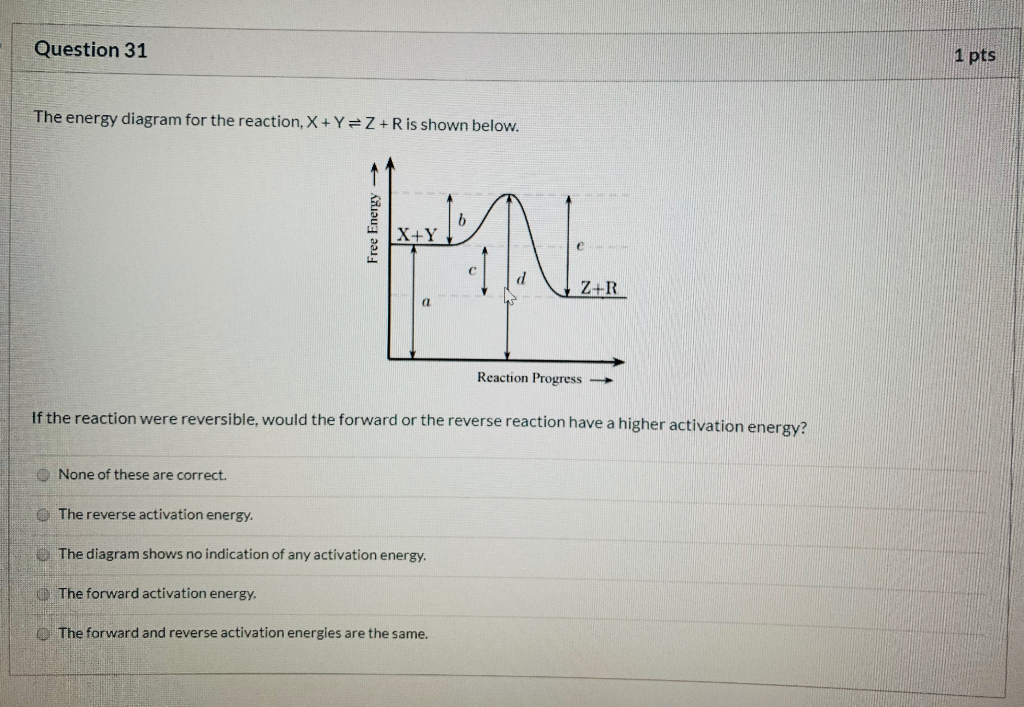
(PDF) Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell... Free-energy diagram for oxygen reduction over Pt(111) based on the energies in Table 1. Results for low obtained by other means. We start by considering the simplest. ∆ E is the reaction energy of eq 6 or 7, ∆ ZPE is the difference. in zero point energies due to the reaction, and ∆ S is the change. PDF Ellingham.fm Ellingham Diagrams. Definitions. The Gibbs free energy (∆G) of a reaction is a measure of the thermodynamic driving force that makes a reaction occur. The reactions are therefore reacting a gas with a condensed phase to make another condensed phase, which reduces the entropy. Kinetics Quiz 4 Potential Energy Diagrams B. Decreasing the ... 4. Consider the following potential energy diagram The Activation energy for the forward reaction is A. 25 kJ B. 50 kJ C. 75 kJ D. 125 kJ 5. Consider the following reaction: ½ H 2(g) + ½ I 2(g) ----- > HI (g) The activation energy for the formation of HI is 167 kJ and for the decomposition of HI is 139 kJ. The PDF CHAPTER 3:Review of Chemical Equilibrium — Introduction The Gibbs energy of the system, G , is the convenient energy function of these state variables The dierence in This gives the Gibbs energy change for the reaction at 25◦C. Equilibrium Composition for Heterogeneous Reactions. We illustrate the calculation of chemical equilibrium when there are...
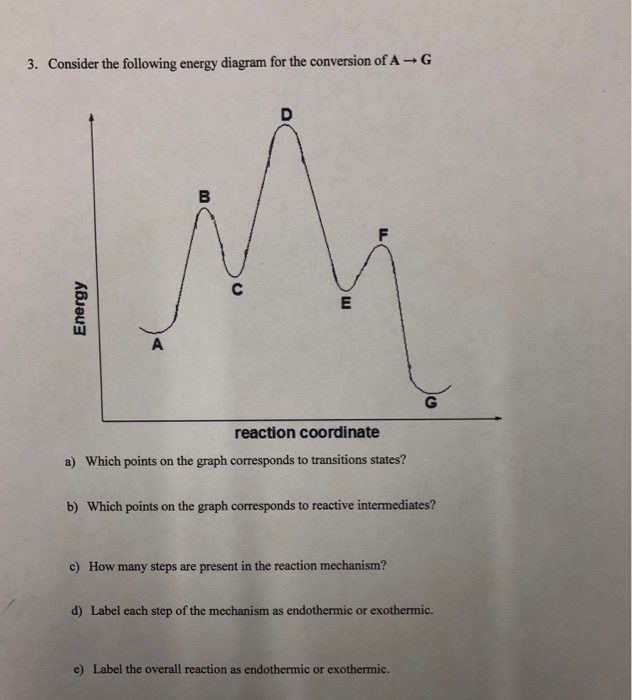
6.10: Describing a Reaction- Energy Diagrams and Transition States interpret the reaction energy diagram for a single-step process (e.g., use the diagram to decide whether the reaction is exothermic or endothermic). suggest possible transition-state structures for simple one-step processes. assess the likelihood of a reaction occurring at room temperature, given...
PDF Slide 1 | Sample Exercise 14.1 Calculating an Average Rate of Reaction Consider a series of reactions having the following energy profiles: Rank the reactions from slowest to fastest assuming that they have nearly the same frequency factors. Solution The lower the activation energy, the faster the reaction. The value of ΔE does not affect the rate.
5.3. Reaction coordinate diagrams | Organic Chemistry 1: An open... In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical SN2 reaction might look like...
Answered: Consider the reaction N₂O(g)… | bartleby Consider the reaction N₂O(g) →N₂(g)1/2O₂(g) with Au has catalyst (a) Is the gold a homogeneous or a heterogeneous catalyst?(b) On the same set of axes, sketch the reaction energy dia-grams for the catalyzed and the uncatalyzed reaction. Q: (a) Draw an energy diagram for the cyclopropenyl MOs.
chemical reaction - Energy considerations | Britannica chemical reaction - chemical reaction - Energy considerations: Energy plays a key role in chemical processes. According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the atoms or pieces of molecules are reassembled into products by forming new...
Презентация на тему: "Chemical Kinetics and Equilibrium. Reaction..." 6 Energy Diagram for Exothermic reaction increasing Average energy energy of reactants Energy difference bet. 9 Surface area of reactants Consider the block of wood or wood shavings The smaller the particle size of wood the greater the surface area exposed to oxygen thus the rate of...
Transition metal Transition metal ions can act as homogeneous catalysts in redox reactions. For example, iron(II) (1) (d) Give one reason why, in the absence of a catalyst, the activation energy for the reaction Draw a diagram to show how the ethanedioate ions are bonded to a copper ion and give a value for one This question is about cobalt chemistry. (a) Consider the following reaction scheme that starts from [Co...
CHEM CH17 HW and NOTES Flashcards | Quizlet For an endothermic reaction, the products will be closer in energy to the transition state than what the reactans will be; so, the activation energy of the reversed reaction is lower than the Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution.
PDF Phase Equilibria, Phase Diagrams and Phase Transformations: Their... 7.1 Molar Gibbs energy diagrams for binary systems 7.2 Instability of binary solutions 7.3 Illustration of the Gibbs-Duhem relation 7.4 Two-phase equilibria in binary systems 7.5 Allotropic phase boundaries 7.6 Effect of a pressure difference on a two-phase. equilibrium 7.7 Driving force for the formation of a...
Gibbs Free Energy | Standard-State Free Energies of Reaction The free energy of a reaction at any moment in time is therefore said to be a measure of the energy available to do work. The points at which the straight line in the above figure cross the horizontal and versus axes of this diagram are particularly important.
Chapter 12 Reaction Rates and Chemical Equilibrium - ppt video... 10 Practice - Energy Diagrams The following reaction is an endothermic reaction: 2 NO2(g) 2 NO(g) + O2(g) 49 Heterogeneous Equilibrium Consider the reaction: Br2(l) Br2(g) 12-. 50 Practice - Equilibrium Constant Expressions Write the equilibrium constant expression for the following reactions.
Chem 12 | D. decreasing the activation energy of the overall reaction. 10. Consider the following potential energy diagram: The above diagram represents an. A. exothermic reaction involving one step. D. potential energy changes into kinetic energy. Consider the following potential energy diagram for the next three five questions.
The diagram shows the free energy change o... | Clutch Prep The diagram shows the free energy change of the reaction. A (g) + B (g) ⇌ AB (g) The reaction progress starts on the left with pure reactants, A and B, at 1 atm and moves to pure product, AB, also at 1 atm on the right. Select the true statements. A. The "x" on the graph corresponds to ΔG of this reaction. B.
PDF Band Structure and Electrical Conductivity in Semiconductors are one of the technologically most important class of materials. According to the band theory of solids, which is an outcome of quantum mechanics, semiconductors possess a band gap, i.e., there is a range of forbidden energy values for the electrons and holes.
Ellingham Diagrams (all content) | Partial pressure of reacting gas We shall consider the following reactions on the Ellingham diagram below: the oxidation of silver to form Ag2O (s); and the oxidation of cobalt to form The reason for the change in slope is the change in phase of a component of the system, which alters the entropy change. As the standard free energy...
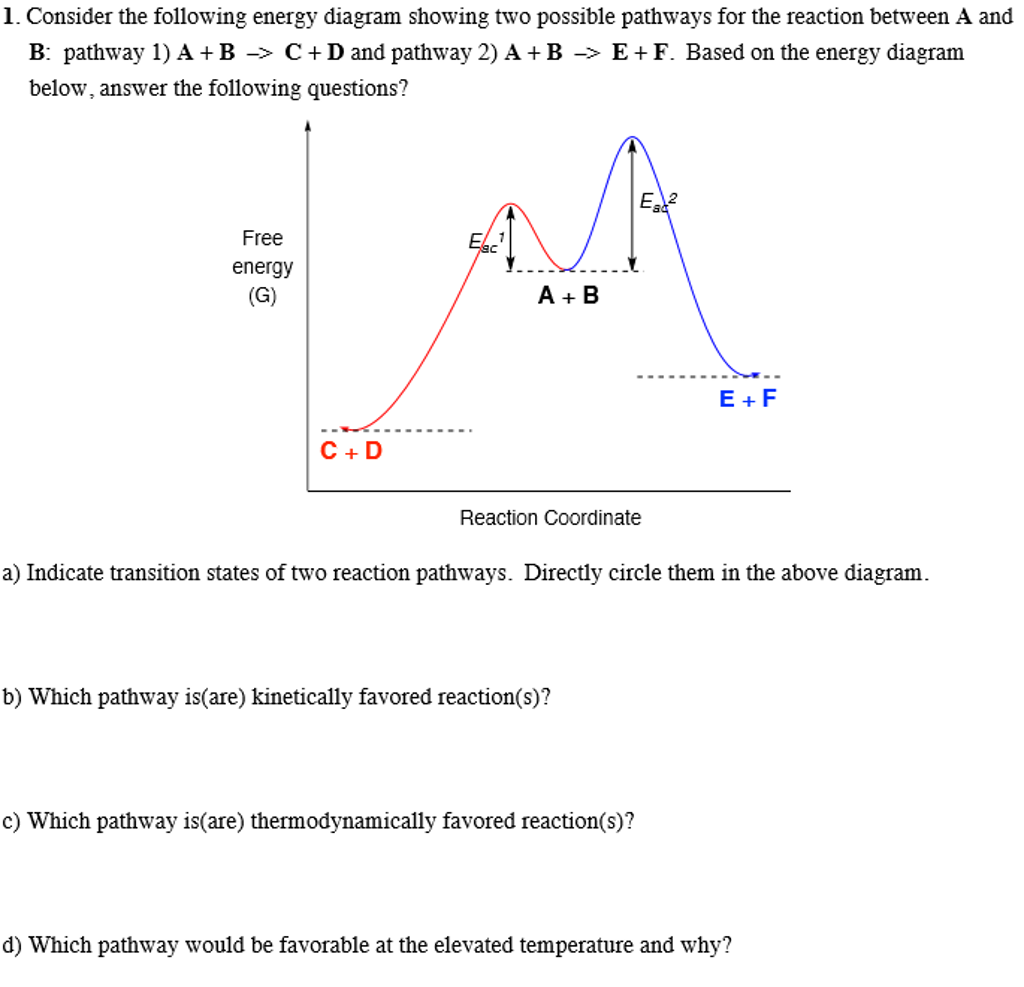
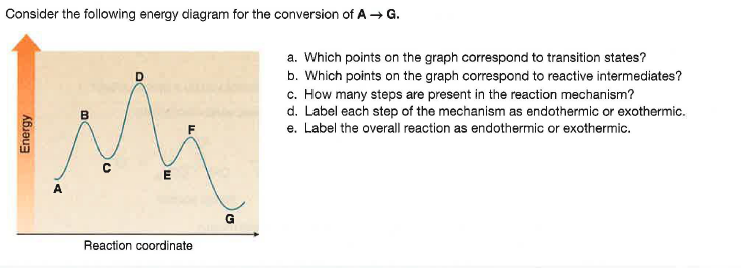
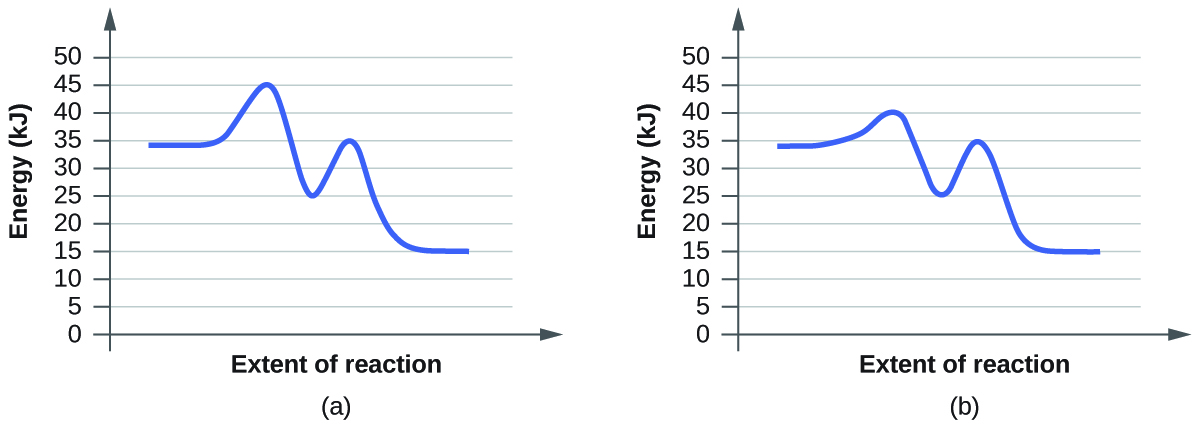
Reaction Mechanisms Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step, and relate this to the activation energy of the Define a chain reaction, and list some of the different kinds of steps such a reaction will involve. Define a branching chain reaction, and explain how such...
PDF Complete the diagram by writing the formulae, including state symbols... (a) The diagram is a Born-Haber cycle for potassium oxide, K2O. The diagram is not to scale and not fully labelled. (i) Complete the diagram by writing the Page 6 of 75. (a) Use this enthalpy change and data from the table to calculate a value for the free-energy change of the reaction at 250 °C. Give...
Solved Consider the energy diagram for the reaction A(g ... Consider the energy diagram for the reaction A(g) rightarrow B(g) Delta E for A(g) rightarrow B(g) 30 kJ/mol The activation energy for A(g) rightarrow B(g) 20 kJ/mol The activation energy for B(g) rightarrow A(g) 50 kJ/mol
PDF Microsoft Word - CallaghanCA_PhDdissertation.doc Figure 7. Typical energy diagram of reaction coordinate as reactants transform to products, overcoming the activation barrier, with a reaction Resistances of the dominant RRs vs. temperature for the following conditions: commercial low temperature shift Cu catalyst loading of 0.14 g/cm3; total...
The diagram for the free energy of the rea... | Clutch Prep Problem: The diagram for the free energy of the reactionA (g) + B (g) ⇌ AB (g)The reaction progress starts on the left with pure reactants, A and B, each at 1 atm and moves to pure product, AB, also at 1 atm on the right. Select the true statements.a. The difference between the top left of the curve and the minimum of the curve corresponds to ...
Chapter 13 Q's Flashcards - Quizlet Consider the following energy diagram and determine which of the following statements is true. At equilibrium, we would expect the concentration of the products to get greater than the concentration of the reactants because the activation energy is lower for the forward reaction.
At a certain temperature, nitrogen and hydrogen react | Chegg.com Transcribed image text: At a certain temperature, nitrogen and hydrogen react to form ammonia: N2(g) + 3H2(g)=2 NH3(g) When initial amounts of N2, H2, and NH3 are OK,>Q OK,«Q OK,-Q OK -Q-O OK, O Consider the free energy diagram below for the reaction: A(g) = B(g). free energy Pure A Pure...




















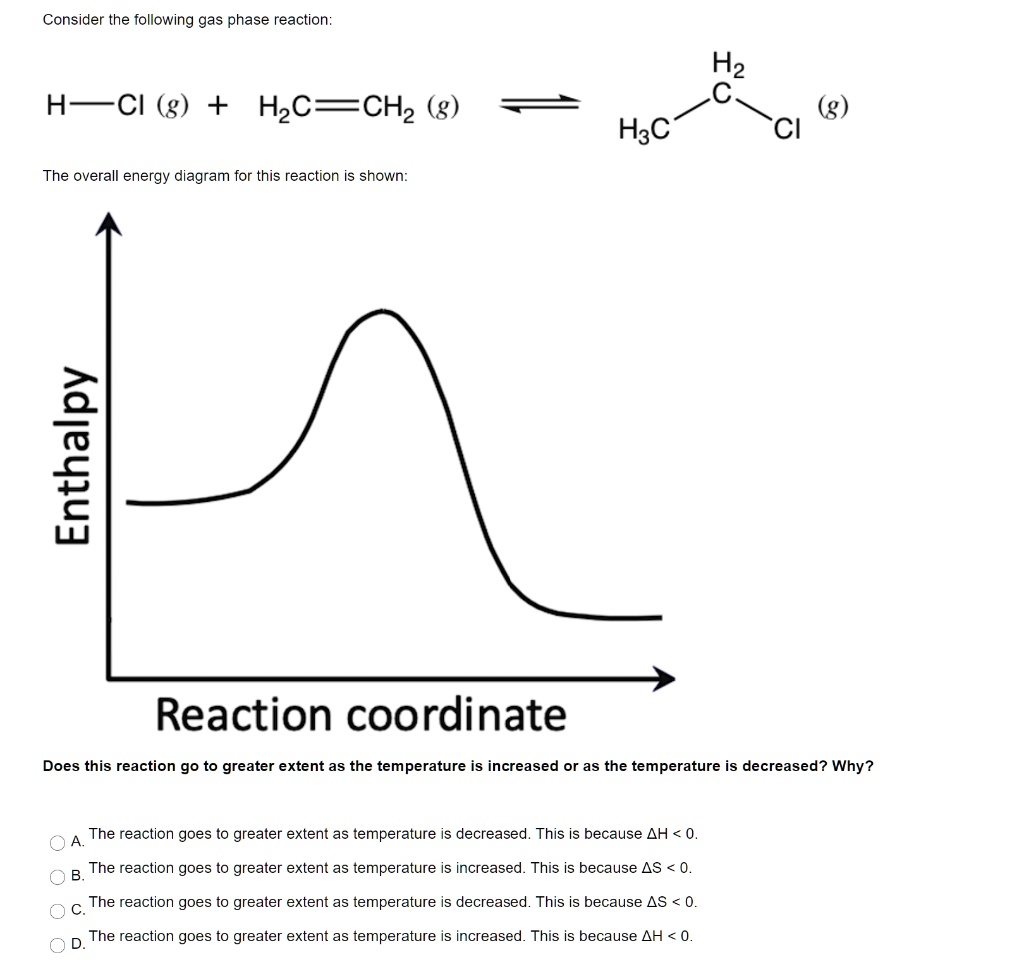



0 Response to "42 Consider The Energy Diagram For The Reaction A(g) B(g)"
Post a Comment