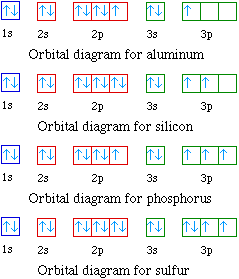
41 orbital diagram for al
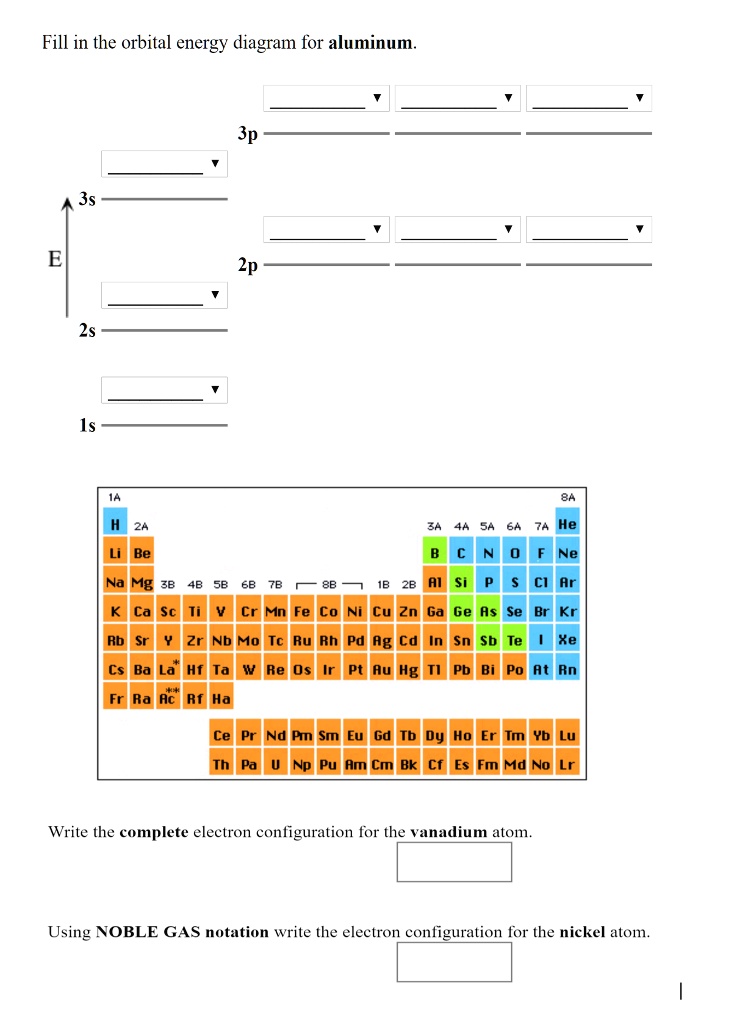
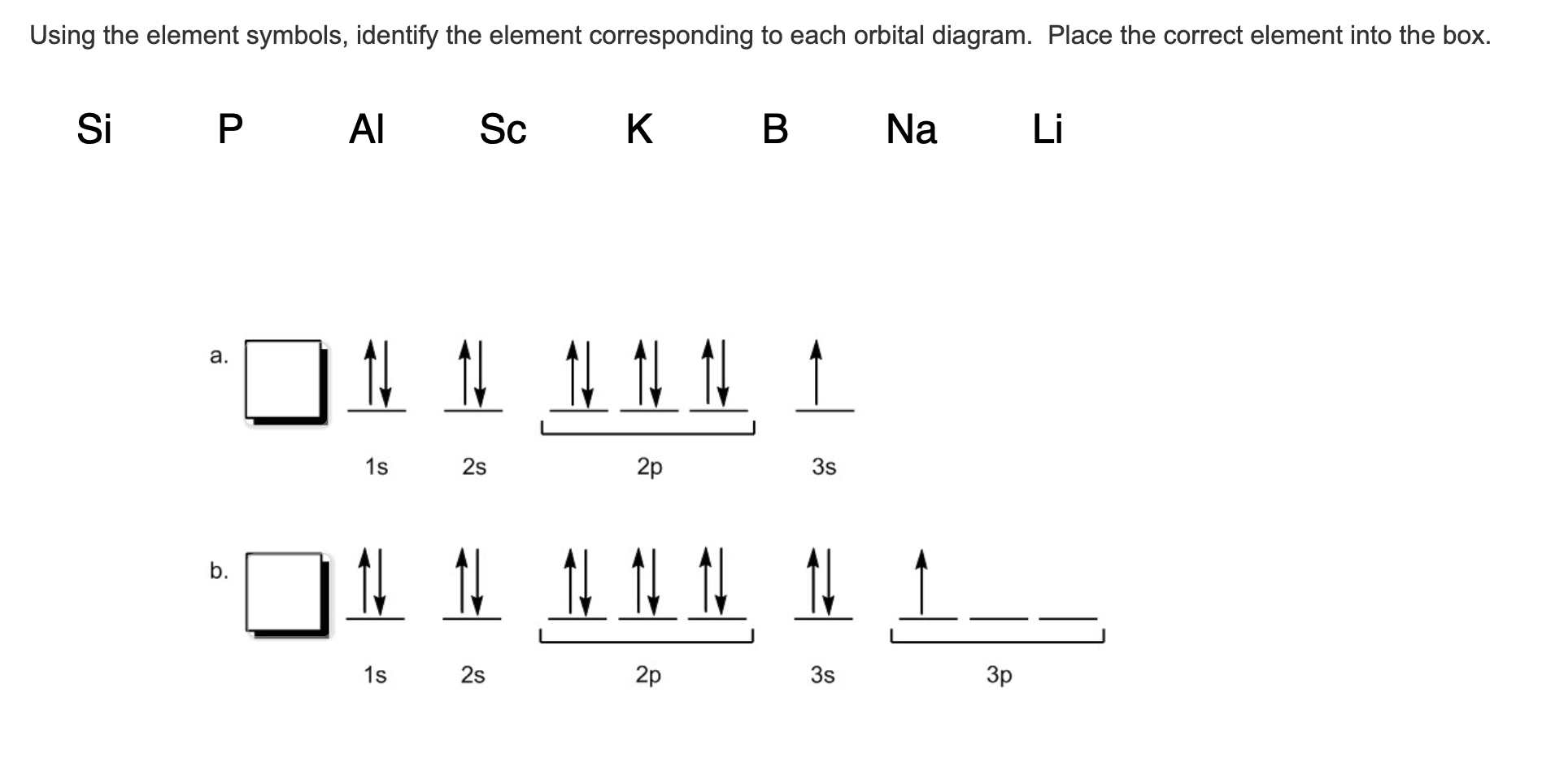
An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. The presenter then goes over the example. Next, the video goes over an ions with a charges S2−, Al3+, and Co2+. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing Aluminum dication loses two electrons Al2+: 1s22s22p63s23p1 = Al2+: 1s22s22p63s1. Draw the orbital diagram for the valence shell of each of the following atoms
If you’re seeking support from others who are sharing common experiences with a spouse, partner or family member struggling with alcoholism, then you may benefit from Al-Anon meetings. Following these guidelines could help you learn how to ...

Orbital diagram for al
Elektronenkonfiguration, [Ne] 3s2 3p1. 1s2 2s2 2p6 3s2 3p1. Orbital Diagramm. Al - Aluminium - Orbital Diagramm - Elektronenkonfiguration ... In this diagram, as in all the orbital diagrams in this book (such as Table 2.3 and Figure 2.6), the signs of orbital lobes are indicated by shading or color. Na MgAl Si 2p Al. P. The result is that the orbital interaction diagram for CO resembles that for a homonuclear diatomic (Figure 5.5), with the... Figure 4 shows a schematic MO diagram of AlSi, com- pared to that of Al 2 . The 3 and 1 orbitals are the bonding MOs between the 3 p orbitals of Al and Si. ... Ionization potentials have been determined and the electronic structures have been discussed within the frame of molecular orbital theory.
Orbital diagram for al. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy Dot diagrams are very different to orbital diagrams, but they're still very easy to understand. They consist of the symbol for the element in the... Partial Orbital Diagrams and Condensed Configurations. A partial orbital diagram shows only the highest energy sublevels being filled. Al has the condensed configuration [Ne]3s23p1. Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(l) Al2O3(s) + 2NaOH(aq) → 2NaAl(OH)4(aq). Only RUB 220.84/month. Orbital Diagrams, Electron Configurations, Electron Configurations, Bohr Models. Al (noble gas configuration).
Presentation on theme: "Draw an orbital diagram for Al"— Presentation transcript 4 Lewis (Electron) Dot diagrams are… A way of showing & keeping track of valence electrons. The symbol represents the nucleus and inner (core) electrons Each dot represents a valence electron (8... Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into What atomic or hybrid orbitals make up the sigma bond between Al and F in aluminum fluoride, AlF3? What is orbital on Al? Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons. Draw the molecular orbital diagram for the oxygen molecule, O2. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here.
Atoms in the same group... 1)have the same outer electron configuration. 2)have the same valence electrons. The group number = How many valence e - in Be Al ... Apr 10, 2021 · Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ... Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following The symmetry occurs because the energies of H(1s) and F(2pz) atomic orbitals are not the same.Molecular orbital diagram for HF... If you have a friend or family member struggling with an alcohol problem, you will often experience your own set of challenges that result from the addiction. Many people affected by someone else’s alcoholism turn to Al Anon for help. Al An...
Similarly, the molecular orbital diagrams for homonuclear diatomic compounds of the alkaline earth metals (such as Be 2 ), in which each metal atom Use a qualitative molecular orbital energy-level diagram to predict the electron configuration, the bond order, and the number of unpaired electrons in...
Al Jubail. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
Aluminum electron configuration. ← Electronic configurations of elements. Al (Aluminum) is an element with position number 13 in the periodic table. Located in ...
If gangster lore sparks your imagination, then Al Capone is probably a name you know quite well. Throughout his life of crime, Capone was responsible for many brutal acts of violence, including the infamous St. Valentine’s Day Massacre that...
In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next ...
In the orbital diagram of carbon, two electrons occupy the 1s orbital, two electrons occupy the 2s orbital, and two electrons each occupy a 2p orbital in the 2p sublevel. The electron-dot symbols for • Groups 1A (1) to 4A (14) use single dots. ·· Na · · Mg ·. · Al · · C ·.
A step-by-step description of how to write the electron configuration for Aluminum (Al). In order to write the Al electron configuration we ...
1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently lower in energy than the valence 5p-orbitals that the valence 5s-orbital of Xe and the 2s-orbitals of the F-atoms form an inner core set.
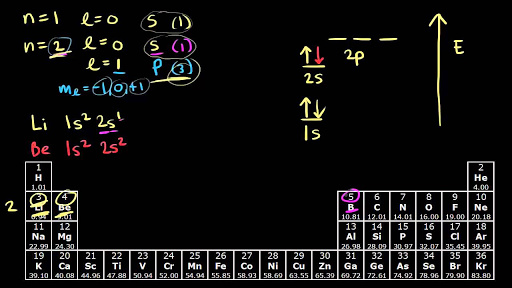
• 3 quantum numbers describing a three dimensional space called an atomic orbital: n, l, m (and spin quantum number describing the electron s) n = principal quantum number, defines the orbital size with values 1 to ∞ l = azimuthal or angular momentum quantum number, defines shape.
We are asked to give the full orbital diagram for aluminum (Al). Aluminum has 13 electrons to distribute because its atomic number is 13 and neutral...
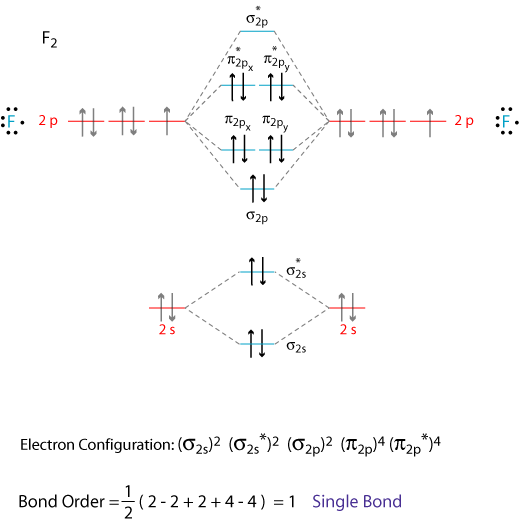
The molecular orbital diagram for dihelium (He2) is the same as that of hydrogen, with the addition of two more electrons (Figure 9.37). Chapter 9. The molecular orbital diagram for the second row homonuclear. Chapter 9. Theories of Chemical Bonding. diatomics (Figure 9.39), therefore shows the...
Element Orbital Diagram. 25 25 35 25 25 25 25 35 . 25 25 35 25 25 25 25 35 . 25 25 35 25 25 25 25 35 . Created Date: 4/21/2011 2:36:22 PM ...
Orbital Diagrams & Electron Configurations for Atoms and Ions Orbital diagrams represent the arrangement of electrons in orbitals. • boxes or lines Orbital filling order for elements beyond Period 2 … ...corresponds to atom's location in periodic table! Draw an orbital diagram for beryllium (Z=4)...
Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as ...
In the Electron Configurations of Main Group Elements lesson, you learned a little bit about valence electrons. You saw how the number and type of valence electrons are important in determining the chemical properties of a particular element.
Alright let's talk about orbital diagrams. Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest...
Orbital Diagrams - . what is an orbital diagram?. an orbital diagram is a representation of an atom where arrows in boxes. Isoelectronic Series • Isoelectronic:having the same number of electrons • N3-, O2-, F-, Ne, Na+, Mg2+, and Al3+ form an isoelectronic series. •
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Orbital Diagrams Chemistry Tutorial. Key Concepts. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence...
Orbitals and molecular representation atomic orbitals. While Lewis diagrams and energy level structures can show connectivity and energy relationships of mol-ecules, they do not show the shape of the molecules.
a. N b. F c. Mg d. Al. Write orbital diagrams for the valence electrons and indicate the number of … Draw the orbital diagram for the valence shell of each of the following atom…
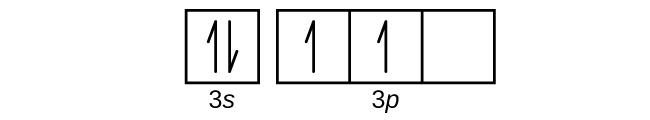
Orbital diagram for aluminum(Al) Electron configuration of aluminum ion(Al 3+) Ground state electron configuration of aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. After the electron configuration, the last shell of the aluminum atom has three electrons. In this case, the valency and valence electrons of aluminum are 3. The elements that have 1, 2, or ...
Schematic molecular orbital diagrams of Al 2 and AlSi. 6 hours ago Al Cu Phase Diagram - Influences Growth Velocity And Fe Content Microstructure electron configurations and orbital diagrams key electron configurations and orbital diagrams key fe 3 1s 2 2s 2 2p 6 3s 3p 6 3d 5...
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p Ionizing the third electron from Al (Al2+ ⟶ Al3+ + e−) requires more energy because the cation Al2+ exerts a stronger pull on the electron than the neutral...
To write the orbital diagram for the Aluminum atom (Al) first we need to write the electron configuration for just Al. To do that we need to ...
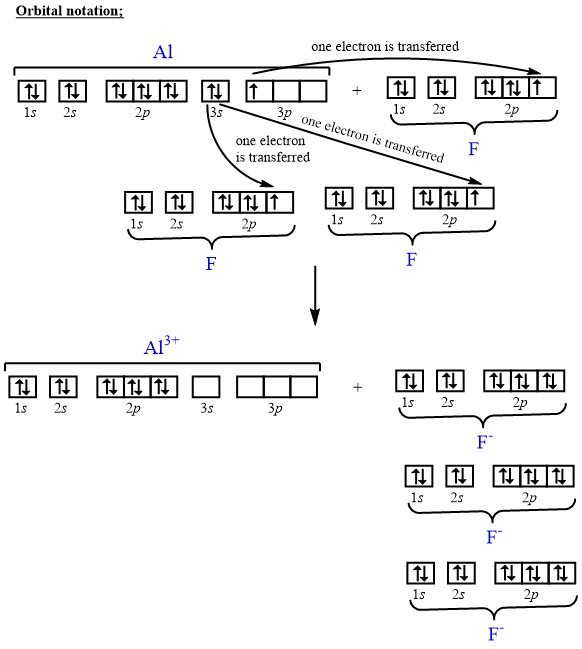
04.08.2016 ... Al3+:1s22s22p6. Explanation: Your starting point here will be the electron configuration of a neutral aluminium atom, Al .
The Orbital Diagram of Aluminium also given here. Aluminium Electron Configuration: Chemical element Aluminium can be written as AL and has atomic number 13. Aluminium is a soft, ductile, magnetic and silver-white element which is from the BORON group.
Figure 4 shows a schematic MO diagram of AlSi, com- pared to that of Al 2 . The 3 and 1 orbitals are the bonding MOs between the 3 p orbitals of Al and Si. ... Ionization potentials have been determined and the electronic structures have been discussed within the frame of molecular orbital theory.
In this diagram, as in all the orbital diagrams in this book (such as Table 2.3 and Figure 2.6), the signs of orbital lobes are indicated by shading or color. Na MgAl Si 2p Al. P. The result is that the orbital interaction diagram for CO resembles that for a homonuclear diatomic (Figure 5.5), with the...
Elektronenkonfiguration, [Ne] 3s2 3p1. 1s2 2s2 2p6 3s2 3p1. Orbital Diagramm. Al - Aluminium - Orbital Diagramm - Elektronenkonfiguration ...
























0 Response to "41 orbital diagram for al"
Post a Comment