41 energy diagram endothermic and exothermic reaction
Dec 01, 2020 · Energy profile diagrams for endothermic and exothermic reactions Every chemical substance has a certain amount of chemical energy. This energy is given the symbol H and is different for different substances. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured. Chemical energy is either released (exothermic reaction) or absorbed (endothermic reaction) during a chemical reaction. In an exothermic reaction, heat is released, creating warmth. In an endothermic reaction, the heat is absorbed, creating cooling. Air bags are activated by a chemical reaction inside
Oct 01, 2019 · A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction. A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction.

Energy diagram endothermic and exothermic reaction
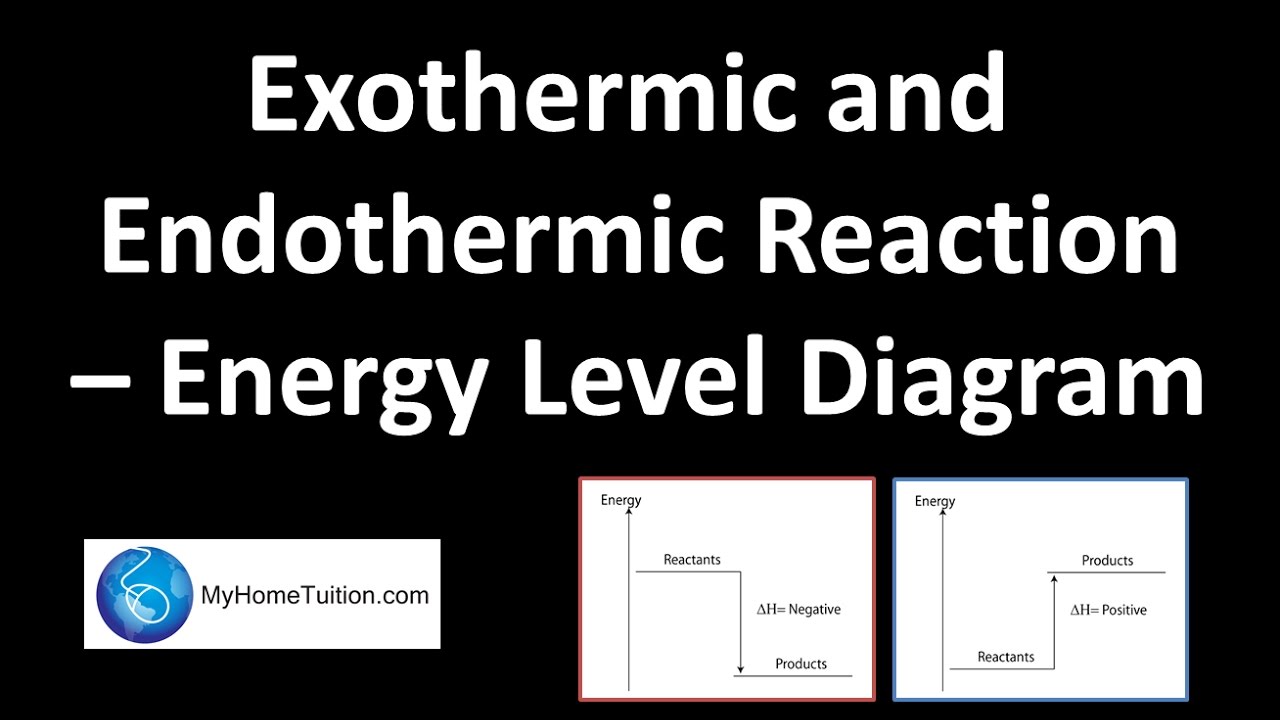
Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > … Nov 23, 2021 · Energy Diagrams Physical or chemical processes can be described through energy diagrams. As mentioned before, reactions can be categorized as endothermic or exothermic processes. The energy diagrams below show what should be known for the test. Image Courtesy of Pinterest Before looking at the specifics of each, you should be aware of a few terms: An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Energy diagram endothermic and exothermic reaction. The reaction shown by the second diagram is more exothermic. There is a greater difference in energy between the reactants and products. The green arrow is longer. In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic. Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
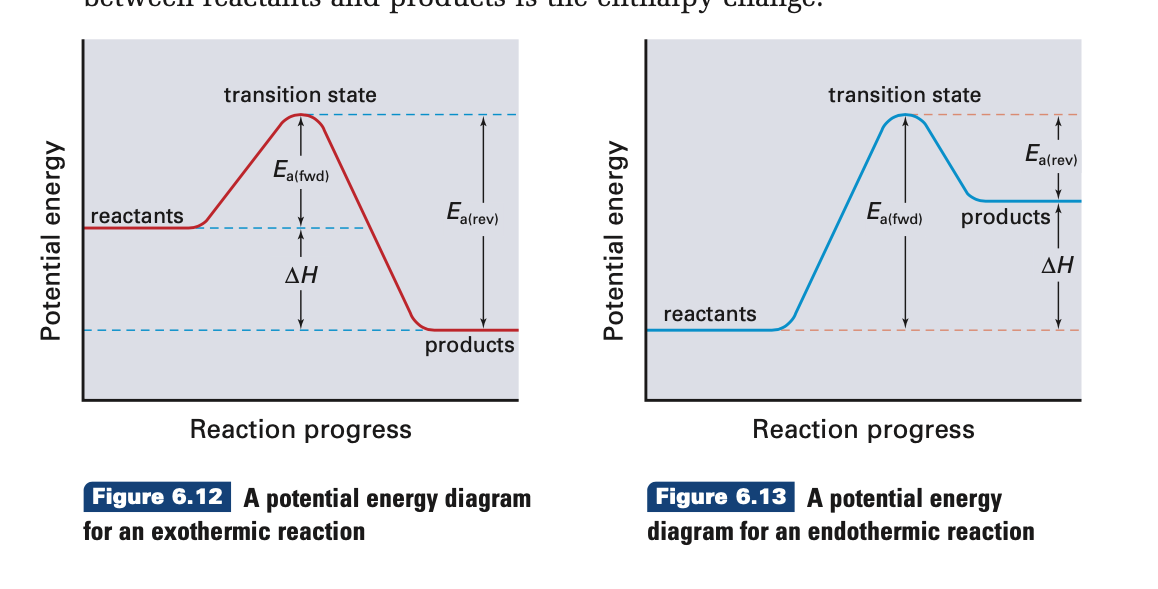
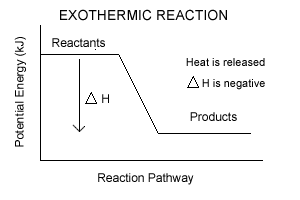
Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ... Exothermic and endothermic reactions When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change. For example, when a bonfire burns it... Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower … DA: 50 PA: 14 MOZ Rank: 17
Similar is the case with the endothermic and exothermic reactions in Chemistry. These release energy in the form of sound, light, cold or heat. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. On the other hand, an exothermic reaction releases energy into the surrounding of the system. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactants. Is freezing water endothermic or exothermic? When water becomes a solid, it releases heat, warming up its surroundings. 5.1 - Exothermic and Endothermic Reactions 5.1.1 - Define the terms exothermic reaction, endothermic reaction and standard enthalpy change of reaction Exothermic Reaction - A reaction that causes the temperature of the surroundings to increase. Energy is lost, or released, in the reaction, as the enthalpy of the products is less
Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
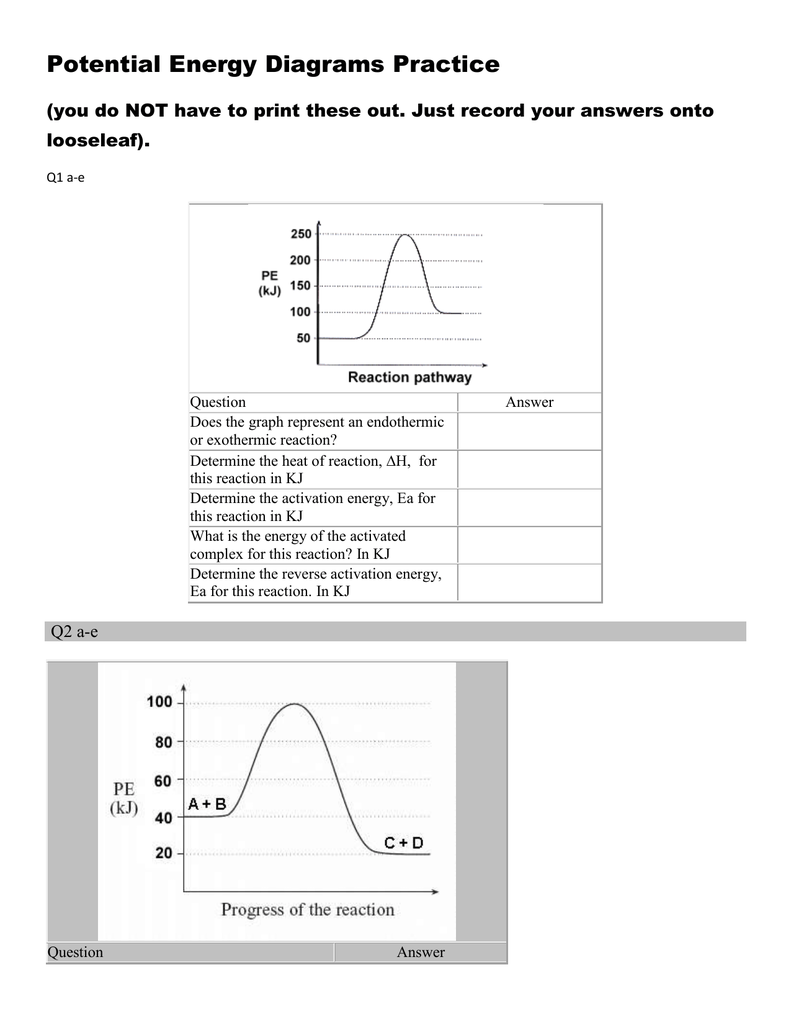
Task 2: Introduction to Energy Diagrams Click here to fill help you fill in this task: Endothermic vs. Exothermic and Energy Diagrams Reference Slides Energy Diagram Shows the changes in potential energy during a reaction. It starts with the _reactants_____ on the left and proceeds to the ____products_____ on the right.
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
The heat of the reaction is absorbed from the surrounding environment. The activation energy for the forward reaction is less than that for the reverse reaction. The energy of the reactants is higher than the energy of the products formed. Heat is released to the surrounding environment. Exothermic reaction Endothermic reaction
Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0).
Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. This means that there was more energy in reactants than in the products.
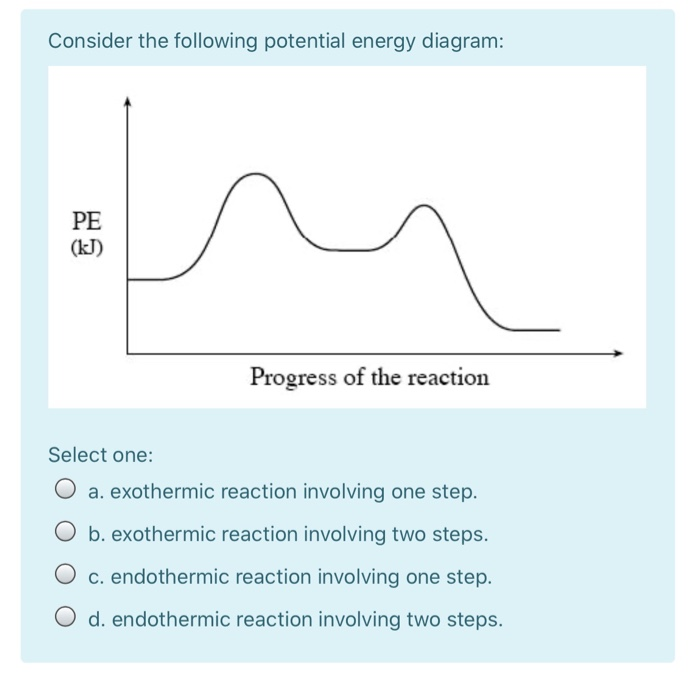
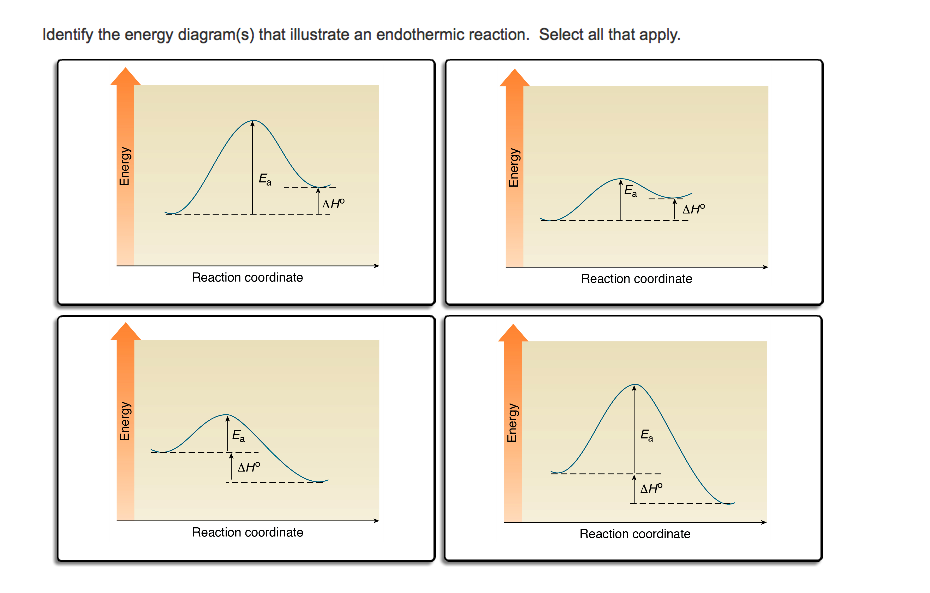
Chemistry. Chemistry questions and answers. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or endothermic?
The major difference between endothermic and exothermic reactions, as their names suggest, is that the former absorbs heat from the surroundings while the latter releases it. Endothermic Reactions – The term “endothermic reaction” refers to a process in which a system absorbs energy in the form of heat from its surroundings.
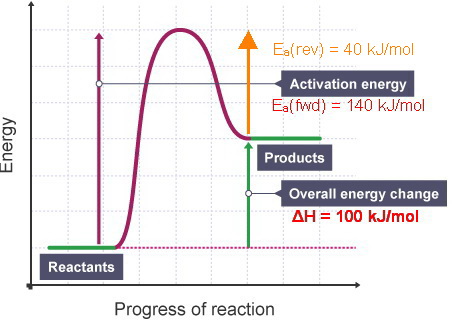
The initial rise in energy represents the energy needed to start the reaction (activation energy Ea) What does a character profile for an endothermic reaction show? The products are at a higher energy than the reactants
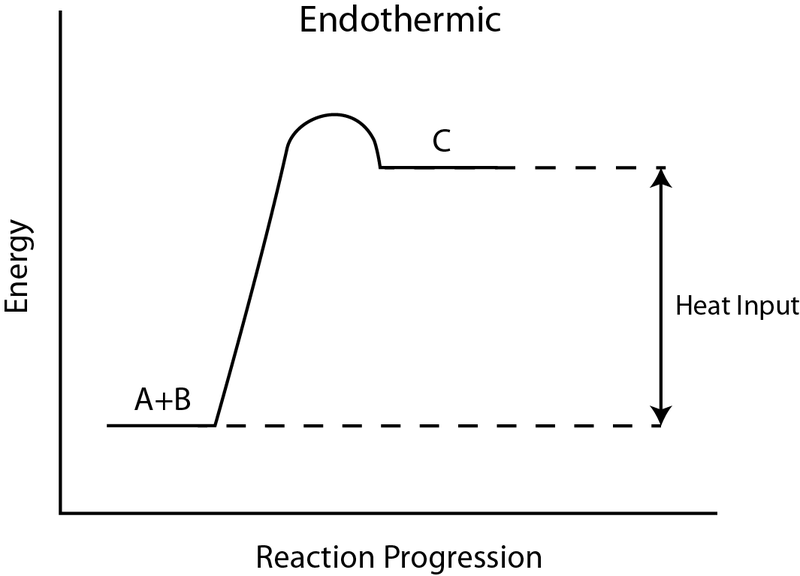
Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic – more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making
Energy must be absorbed to break a bond, so breaking bonds is endothermic. Making new bonds is exothermic because energy is released. a) When green copper carbonate decomposes, the equation is: CuCO 3 CuO CO 2 copper carbonate copper oxide carbon dioxide Is the reaction exothermic or endothermic? Use ideas about bonds to explain why.
An endothermic phase change absorbs heat or energy, while an exothermic phase change releases heat or energy, states the washington university department of chemistry. the following are additional endothermic phase changes: fusion, or melting, when a solid transforms to liquid. vaporization, when a liquid transforms to gas.
Use these examples to classify the changes in energy of a system as exothermic or endothermic. Learn with flashcards, games, and more — for free.
Endothermic and exothermic reactions Paul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.
The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react.
Powered by FlexBook® textbook Platform ® © CK-12 Foundation 2022; Please wait... Please wait...
Energy is absorbed to break bonds. Bond-breaking is an endothermic process. Energy is released when new bonds form. Bond-making is an exothermic process. Whether a reaction is endothermic or ...
Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure PageIndex2. As we can see and examine from the graph in an exothermic reaction the reactants are usually at a higher. In this type of reaction energy in the form of heat sound or light is released when the reactants break apart.
Solids and Liquids, Endothermic and Exothermic, Le Chatelier A second side note about K is that pure solids and pure liquids don't get included in it. Their concentrations are so big that we can just take them as being constant throughout the reaction.
Exothermic reactions release energy to their surroundings, because the products are lower in energy than the reactants. You can think about this visually using a reaction energy diagram, as seen below: And endothermic reaction (left) and an exothermic reaction (right) plotted on a plot of energy against the reaction coordinate (a measure of the ...
An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Nov 23, 2021 · Energy Diagrams Physical or chemical processes can be described through energy diagrams. As mentioned before, reactions can be categorized as endothermic or exothermic processes. The energy diagrams below show what should be known for the test. Image Courtesy of Pinterest Before looking at the specifics of each, you should be aware of a few terms:
Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > …























0 Response to "41 energy diagram endothermic and exothermic reaction"
Post a Comment