40 h2 molecular orbital diagram
This video discusses how to draw the molecular orbital (MO) diagram for the H2(2+) molecule. The bond order of H2(2+) is calculated and the meaning of this n... Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
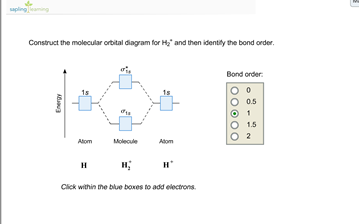
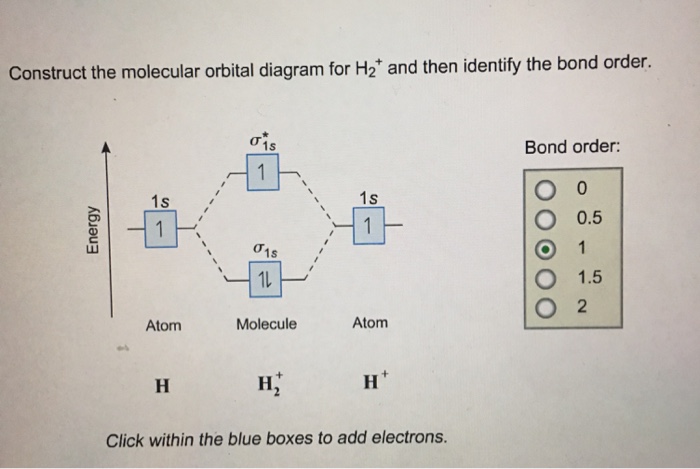
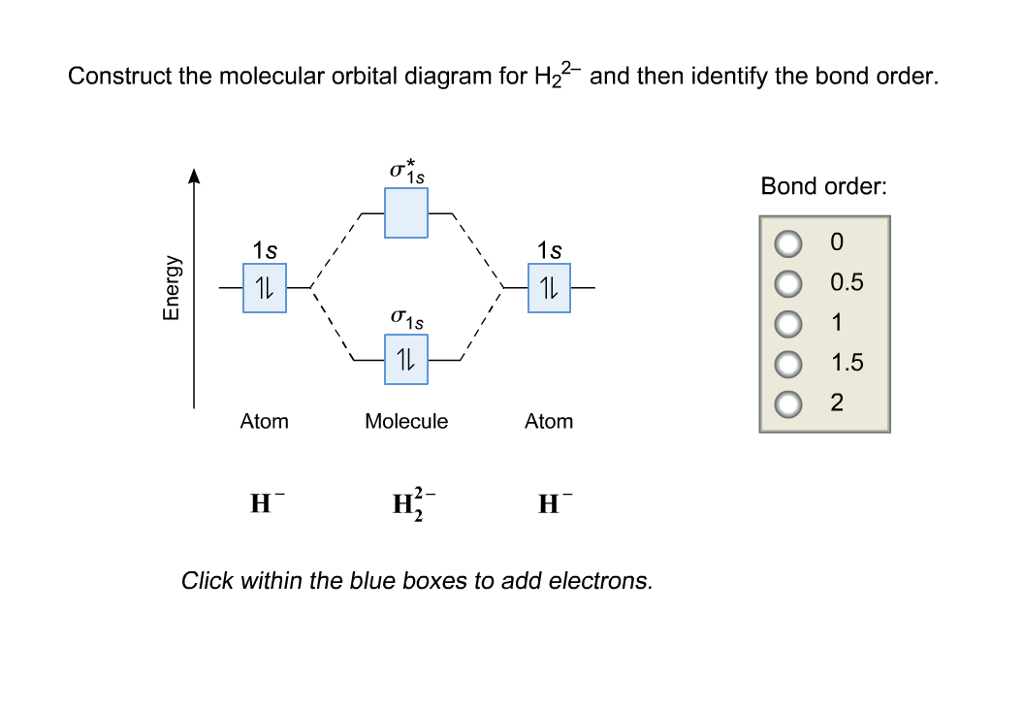
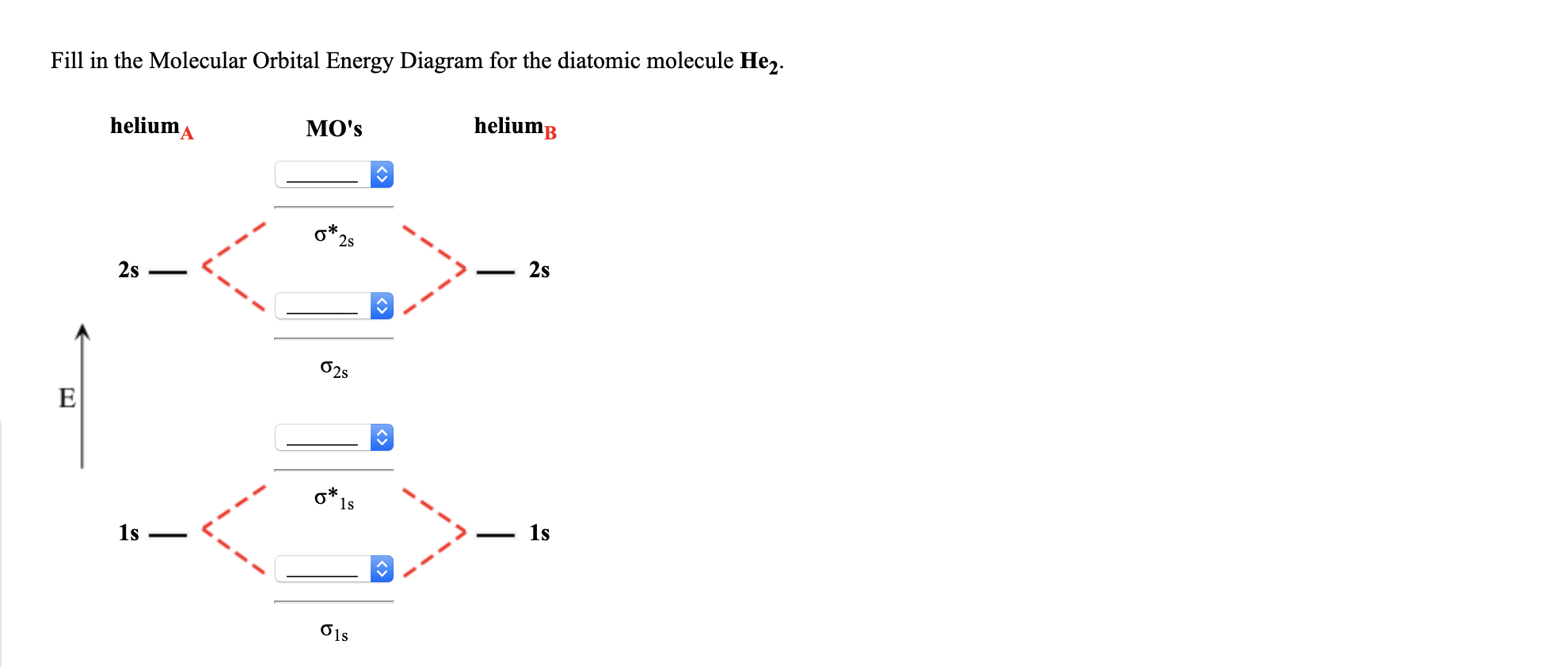
1. Construct the molecular orbital diagram for H2+2, H2+, and H2- and find there bond orders in decimal form. part b of question 6 what is the bond order of Be2+? also numerical.
H2 molecular orbital diagram
Answer (1 of 2): According to the molecular orbital theory, a molecular is viable of its bond order is more than or equal to one. The bond order is defined as number of bond between to two atoms of that molecule. It is calculated as the difference of electrons in bonding molecules and anti-bondin... In the case of H 2 both of the valence electrons that form the bond between the hydrogens fill the bonding or s orbital. Principle 2 & 3: This interaction of atomic orbitals, which gives rise to the molecular orbitals, may also be represented in the form of an orbital (electron) energy diagram which shows the relative energies of the orbitals. The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
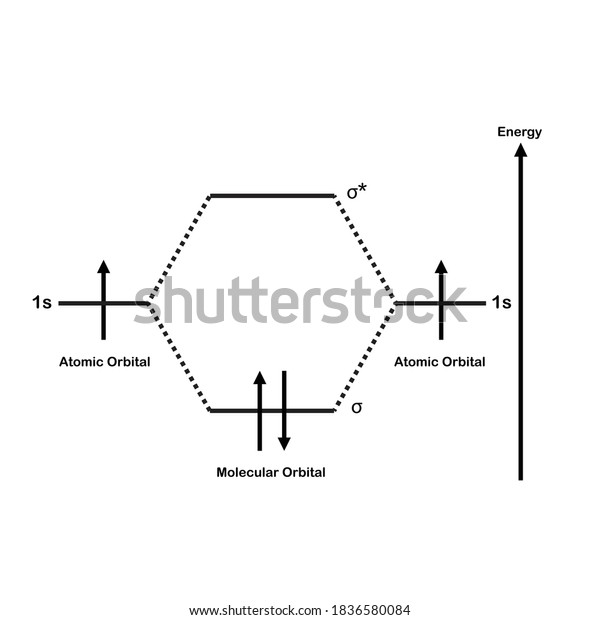
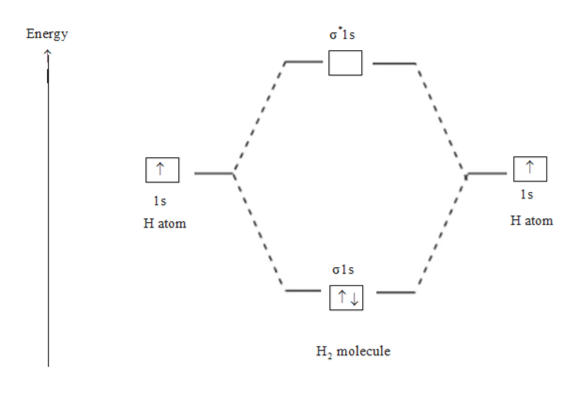
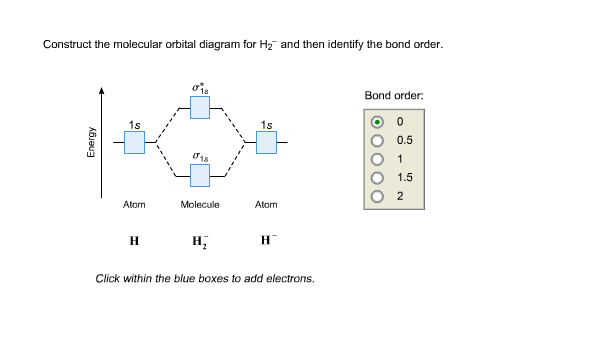
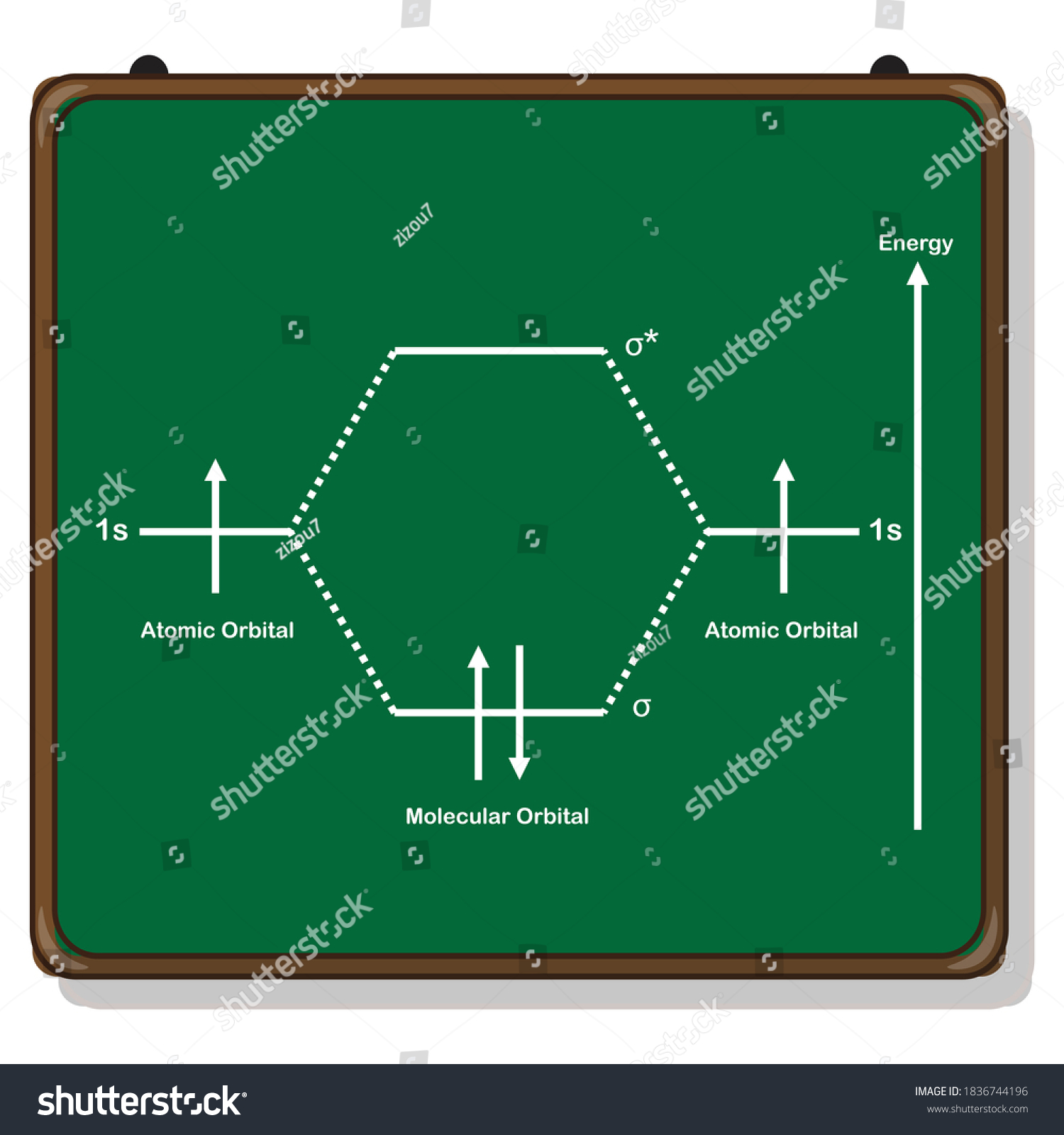
H2 molecular orbital diagram. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Dec 15, 2018 · Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with magnetic behavior and bond order. Verified. 82.1k+ views. Hint: Generally the molecular orbital diagrams are used to understand the bonding of a diatomic molecule. You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to ...
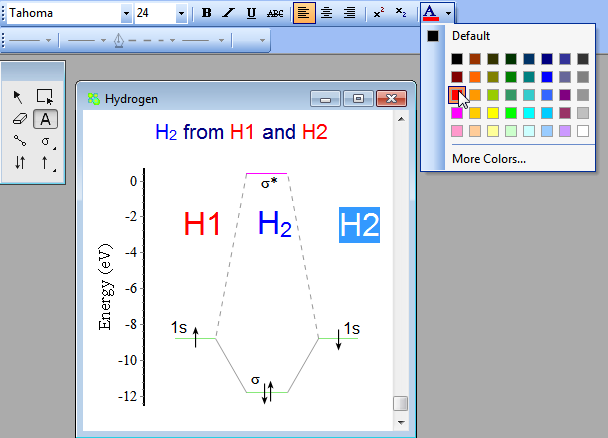
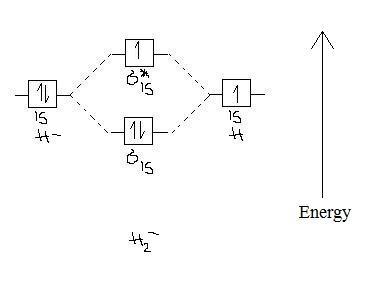
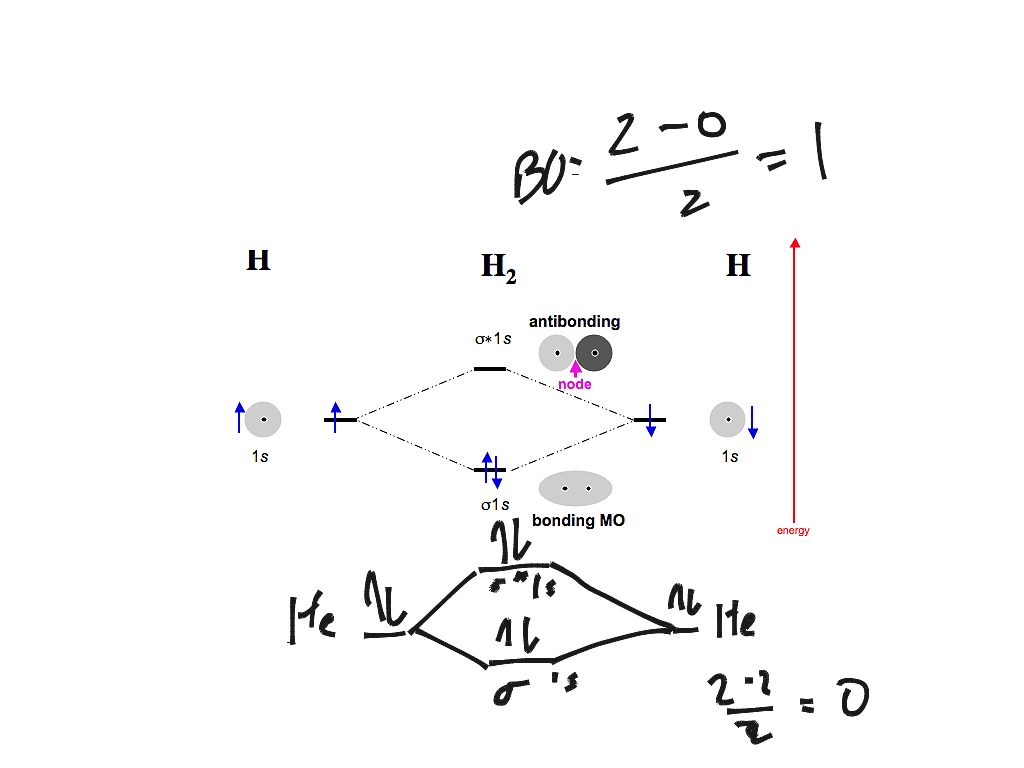
Answer (1 of 4): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi... starting with the lowest energy molecular orbital. The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy, or bonding, molecular orbital, as shown in the figure below. This diagram suggests that the energy of an H2molecule is As a result, the H2molecule is more stable than a pair of isolated atoms. LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 •∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. HaHb MOs for H2 for covalent bonding. Formation of molecular orbital diagram is given with diagram. Combinations of s-s, s-p, p-p, p-d orbitals. Molecular orbital energy level diagram for H2+, H2, He2+, He2, Li2, Be2, B2, C2, N2, O2, F2, CO, NO, HCl, CO2, NO2 were explained with suitable diagram in six steps. Page 4/30
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals. Answer (1 of 4): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi... The molecular orbital energy-level diagram shown in Figure 13 also applies (with changes of detail in the energies of the molecular orbitals) to the hypothetical species He 2. However, this species has four valence electrons, and its configuration would be 1σ 2 2σ 2. brings only a 1s orbital to the mixing. Just as in the molecules H2 and H2 +, the molecular orbitals for He2 are created by the combination of two 1s atomic orbitals (Fig. 1.46). Each helium atom brings two electrons to the molecule, though, so the electronic occupancy of the orbitals will be different from that for H2 or H2 +. The
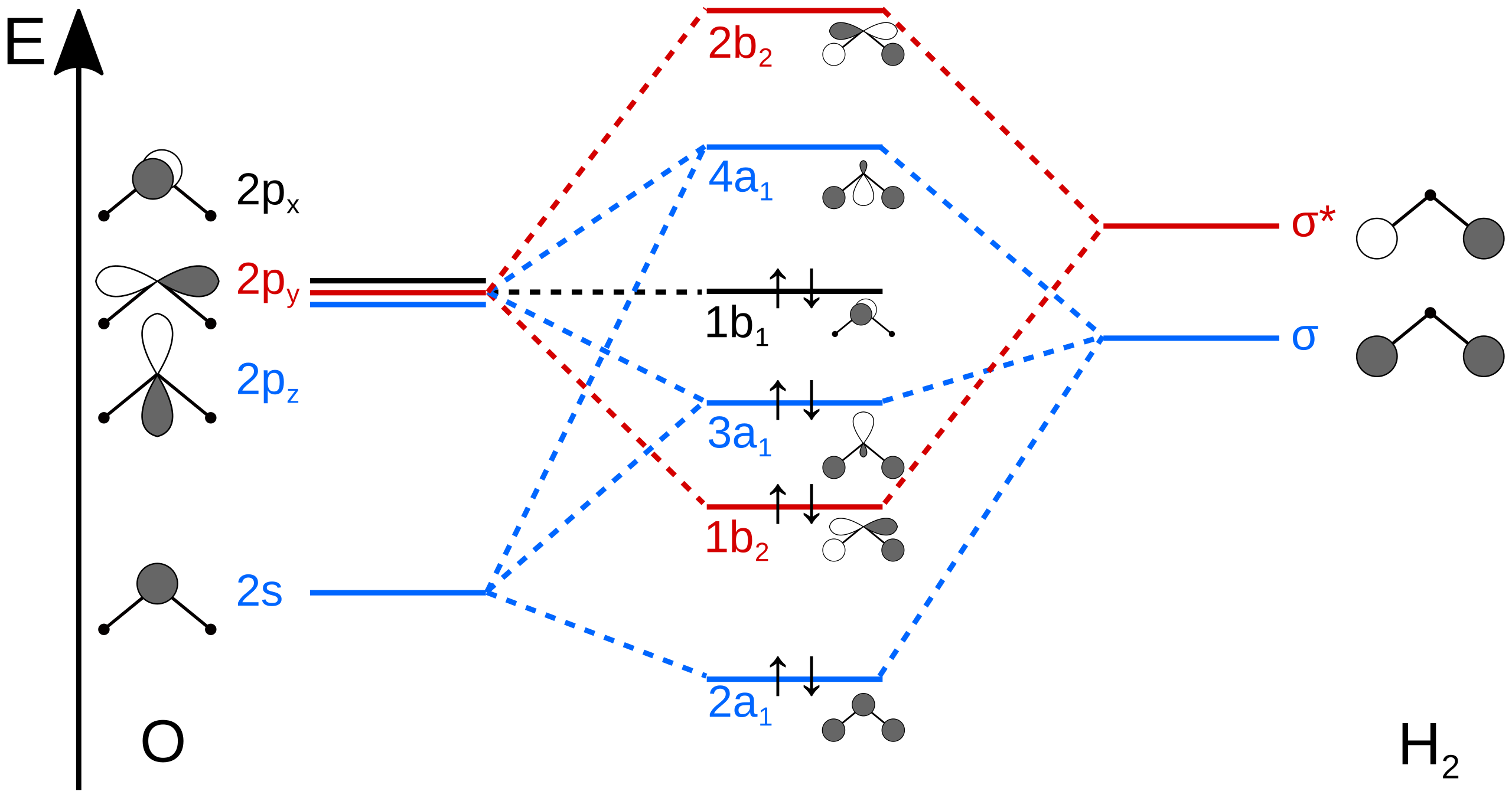
The head-to-head overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10").
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules bond order = (number of bonding electrons) - (number of antibonding elect rons) 2 = amount of bonding 1sa hydrogen molecule = H2 LUMO HOMO σ = 1sa + 1sb = bonding MO = potential energy higher, less stable lower, more stable LUMO = lowest unoccupied molecular orbital HOMO = highest ...
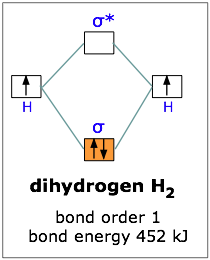
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
Question: Each of the following five statements concerns the molecular orbital diagram for H2-. Indicate whether each statement is true or false. This problem has been solved! See the answer See the answer See the answer done loading.
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
of the orbitals is specified. b. Molecular Orbital Picture We are now in a position to discuss the basic principles of the molecular orbital (MO) method, which is the foundation of the electronic structure theory of real molecules. The first step in any MO approach requires one to define an effective one electron Hamiltonian, hˆ eff. To this ...
In H2, we have 2 hydrogen atoms, each with a 1s orbital. These orbitals are pointing at each other along the z axis, so they will make sigma orbitals. We can make molecular orbitals by combining these 2 atomic orbital to obtain 2 molecular orbitals. One orbital comes from addition, {H11s + H21s}, and the other comes from subtraction, {H11s - H21s}.
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic ... Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can “mix” (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ ...
The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
In the case of H 2 both of the valence electrons that form the bond between the hydrogens fill the bonding or s orbital. Principle 2 & 3: This interaction of atomic orbitals, which gives rise to the molecular orbitals, may also be represented in the form of an orbital (electron) energy diagram which shows the relative energies of the orbitals.
Answer (1 of 2): According to the molecular orbital theory, a molecular is viable of its bond order is more than or equal to one. The bond order is defined as number of bond between to two atoms of that molecule. It is calculated as the difference of electrons in bonding molecules and anti-bondin...



















0 Response to "40 h2 molecular orbital diagram"
Post a Comment