38 no2- molecular orbital diagram
Hybridization of NO2 - Hybridization of N in Nitrogen Dioxide Hybridization of NO2 (Nitrogen Dioxide) NO 2 involves an sp 2 type of hybridization. The most simple way to determine the hybridization of NO 2 is by drawing the Lewis structure and counting the number of bonds and lone electron pairs around the nitrogen atom. You will find that in nitrogen dioxide there are 2 sigma bonds and 1 lone electron pair. Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
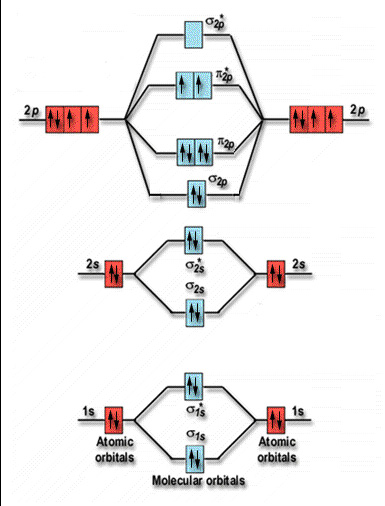
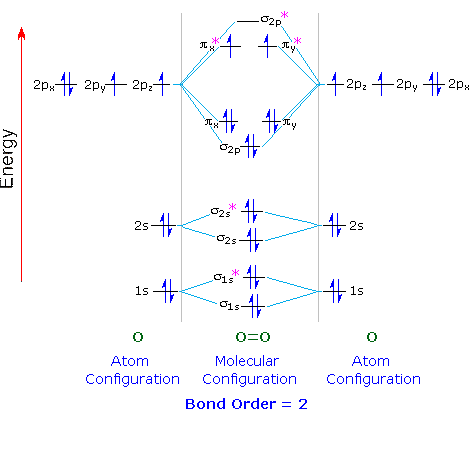
Explain the formation of nitrogen molecule by molecular ... Nitrogen molecule (N 2) The electronic configuration of nitrogen (Z=7) = 1s 2 2s 2 2p x1 2p y1 2p z1. The total number of electrons present in the nitrogen molecule (N 2) is 14. In order to maximize energy, these 14 electrons can be accommodated in the different molecular orbitals. N 2: KK' (σ2s) 2 (σ*2s) 2 (π2Px) 2 (π2py) 2 (σ2pz) 2.
No2- molecular orbital diagram
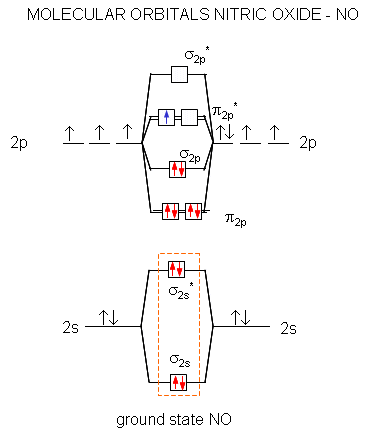
37 no+ molecular orbital diagram - Diagram For You 37 no+ molecular orbital diagram. Mar 18, 2018 · "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram. By writing molecular orbital configuration for NO,CO,O2 ... #"O"_2# is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that #"CO"# is not (as it has zero unpaired electrons), but #"NO"# is (it has one unpaired electron). Well, the MO diagram for #"O"_2# is: The bond order is already calculated in the diagram. PDF Polyatomic Molecular Orbital Theory - La Salle University Polyatomic Molecular Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry. • Symmetry properties and degeneracy of orbitals and bonds can be ...
No2- molecular orbital diagram. NO2 Lewis Structure: Complete Guide (2022 Updated) Molecular Geometry. According to the VSEPR theory, The molecular structure of NO2 (Nitrogen Dioxide) is bent. However, there are a few exclusions in this case. There are two bonded pairs and an unpaired electron in the Lewis structure for NO2. When we examine the nitrate ion NO2-, we notice that it contains two bond pairs, one lone pair of ... draw the molecular orbital diagram of n2 also find its ... Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule. Answered by | 13th Jun, 2016, 04:45: PM. Concept Videos. Molecular Orbital Theory - Part 1. Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or... Molecular Orbital Diagram For Ne2 Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
Bonding in Nitrogen and Related Molecules In both molecules the pi symmetry molecular orbitals are the same. The 2p x orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane. Perpendicular to these in the yz plane, the 2p y orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital. Here is the full molecular orbital diagram for N 2. No2 Molecular Orbital Diagram — UNTPIK APPS No2 Molecular orbital Diagram. molecular orbital theory structure of no2 pound the electron population of this orbital is see table vi 0 53 on the n atom 0 16 2s 0 37 2pz 0 24 on each o atom 0 24 pz experimentally oxides and oxyions of the non metals part ii c02 and no2 of the chemical society 1962 2873 2880 collects values for the partition of unpaired electron density among n and o atomic ... The hybridization of orbitals of N atom in NO3-, NO2+ and ... NO2+Number of electron pairs = 2Number of bond pairs = 2Number of lone pair = 0So, the species is linear with sp hybridisation.NO3-Number of electron pairs = 3Number of bond pairs = 3Number of lone pair = 0So, the species is trigonal planar with sp2 hybridisation NH4+Number of electron pairs = 4Number of bond pairs = 4Number of lone pair = 0So, the species is tetrahedral with sp3 hybridisation. molecular orbital theory - Structure of NO2 compound ... According to Electronic Structure of NO2 Studied by Photoelectron and Vacuum-uv Spectroscopy and Gaussian Orbital Calculations J. Chem. Phys. 53, 705 (1970) : The highest molecular orbital 4 a 1 is occupied by the 1 unpaired electron. Experimentally, Oxides and Oxyions of the Non-metals.
Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... Draw molecular orbital diagram of O2 or N2 with magnetic ... As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [ N b − N a ] / 2 = [ 1 0 − 6 ] / 2 = 2 . Consider the molecular orbital energy leve... | Clutch Prep Consider the molecular orbital energy level diagrams for O2 and NO2. Which of the following is true? I. Both molecules are paramagnetic. II. The bond strength of O2 is greater than the bond strength of NO. III. NO is an example of a homonuclear diatomic molecule. IV. The ionization energy of NO is smaller than the ionization energy of NO^+. A ... Molecular Structure Practice Problems Answers The molecular orbital diagram for ClO - is given below: The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated.
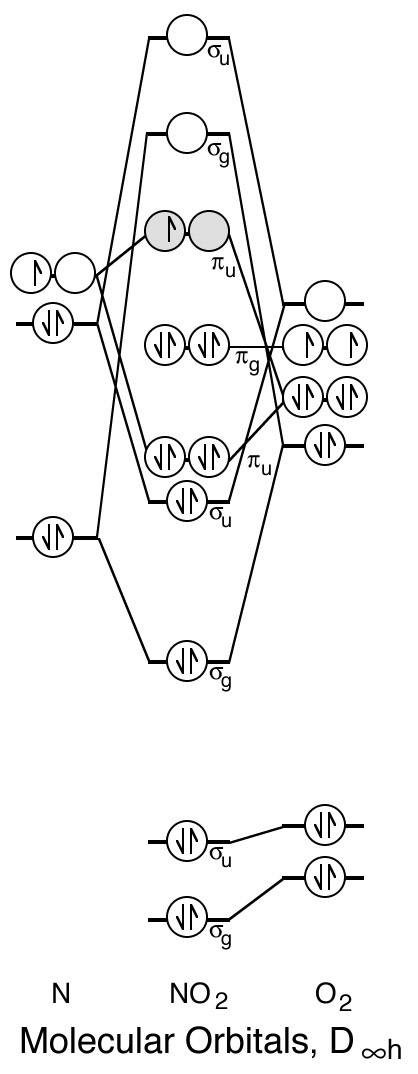
N2O4 Lewis Structure, Molecular Geometry, Hybridization ... N2O4 Molecular Orbital (MO) Diagram. A molecular orbital (MO) diagram explains the chemical bonding in molecules by energy level diagrams. They were proposed by Robert S. Mulliken and Friedrich Hund in 1928. As we know N2O4 molecule is a dimer of the NO2 molecule, hence we'll discuss molecular orbital diagram of NO2 molecule first.
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
40 molecular orbital diagram for n2 - Wiring Diagram Source Molecular orbital diagram for n2. No2 Valence Electrons - 9 images - solved question 9 of 13 determine the number of valence e, ppt chemical bonding powerpoint presentation free, Shouldn't you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence ...
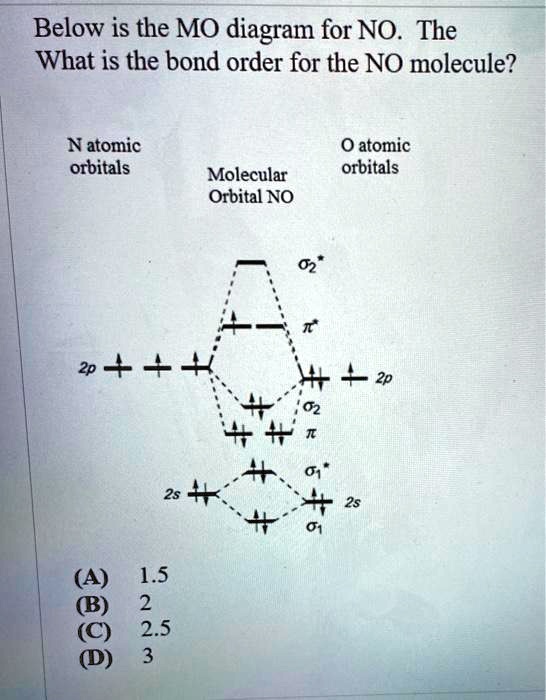
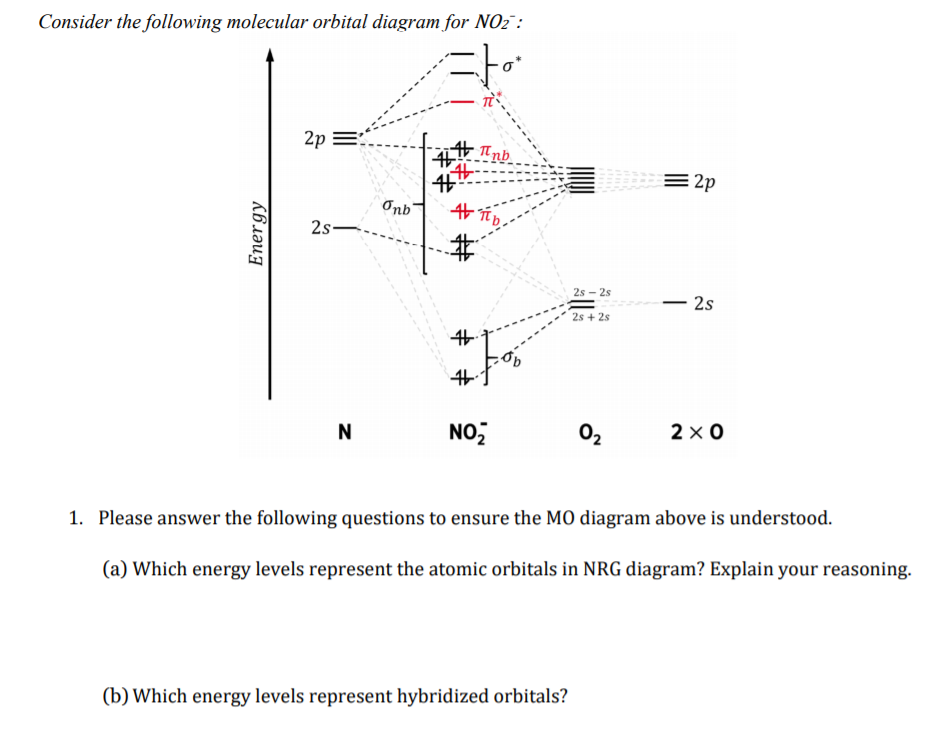
Solved Consider the following molecular orbital diagram ... Solved Consider the following molecular orbital diagram for | Chegg.com. Science. Chemistry. Chemistry questions and answers. Consider the following molecular orbital diagram for NO2 : 2p ппы 41 = 2p Onb Energy 2s 46 # # 2s - 2s 2s 2s + 2s # N NOŻ 02 2 x 0 1. Please answer the following questions to ensure the MO diagram above is understood.
NO2 Lewis Structure, Molecular Geometry, Hybridization ... VSEPR chart: We can see that NO2 has a bent molecular geometry and the angle is around 120 degrees. But here we have some exceptions. In NO2, we have 2 Bond Pairs and 1 lone electron. If we look at the nitrite ion NO2-, we have 2 Bond Pairs and 1 Lone pair of electrons.
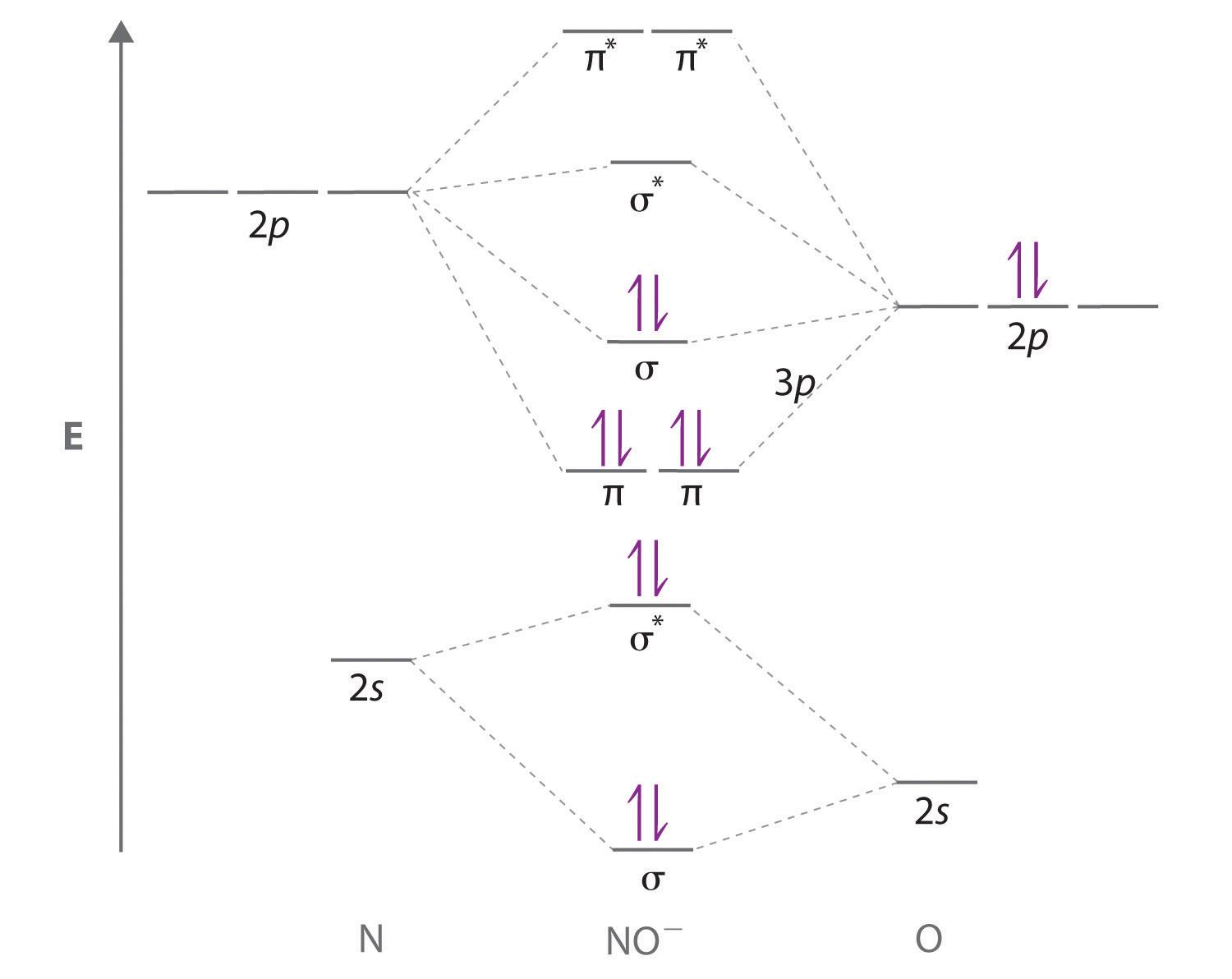
What is the molecular orbital diagram for NO₂? - Quora Answer: I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion (NO₂¯) and then remove an electron from it: What will be the molecular orbital diagram for nitrite ion? The outcome, i.e. the molecular orbital diagram for Nitrogen dioxide NO₂, should loo...
Solved NO2+ molecular orbital diagram | Chegg.com This problem has been solved! See the answer. See the answer See the answer done loading. NO2+ molecular orbital diagram. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (1 rating)
Draw and explain the molecular orbital diagram for NO2 ... The molecular orbital diagram (MOT) is useful to predict bond order, bond strength, bond energy, stretching frequency, and bond length. Bond order has direct links with bond strength, bond energy ...
Nitrogen Dioxide - Beloit College Molecular orbitals in NO 2 Will the molecule be linear or bent? Click on a color picture to watch the geometry change from linear to bent. 2흅u: 2b 1 6a 1 : 1흅g: 1a 2 4b 2 : 1흅u: 1b 1 5a 1: Movies on this page were created as linear combinations of atomic orbitals by George Lisensky, Beloit College.
Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
PDF Polyatomic Molecular Orbital Theory - La Salle University Polyatomic Molecular Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry. • Symmetry properties and degeneracy of orbitals and bonds can be ...
By writing molecular orbital configuration for NO,CO,O2 ... #"O"_2# is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that #"CO"# is not (as it has zero unpaired electrons), but #"NO"# is (it has one unpaired electron). Well, the MO diagram for #"O"_2# is: The bond order is already calculated in the diagram.
37 no+ molecular orbital diagram - Diagram For You 37 no+ molecular orbital diagram. Mar 18, 2018 · "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram.




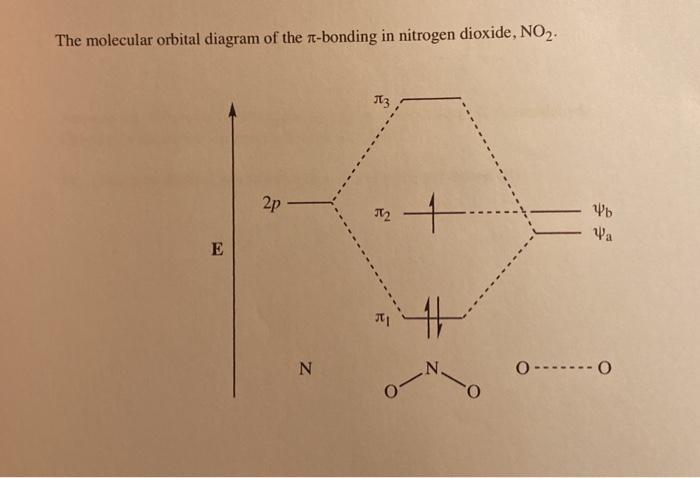




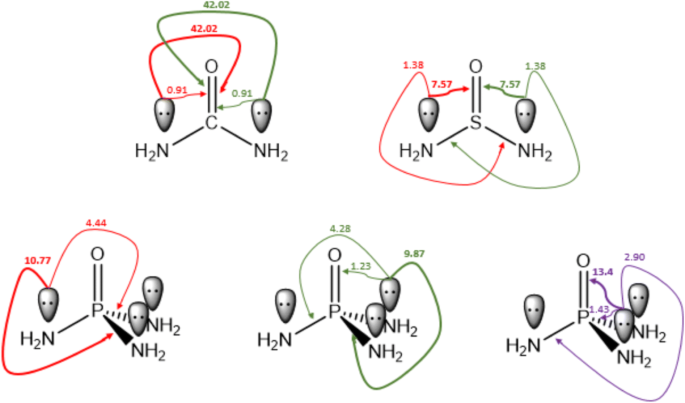
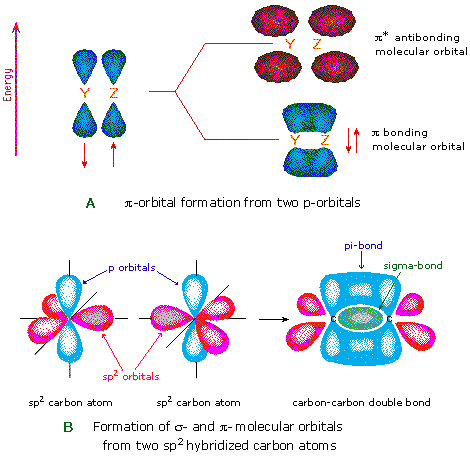







0 Response to "38 no2- molecular orbital diagram"
Post a Comment