38 in an electron dot diagram of ethylene how many double bonds are present
In an electron dot diagram of ethylene (C2H4) how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron. Which of the following intermolecular forces plays a pivotal role in the unique properties of water? OR We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations.
05.01.2022 · Semiconductor superstructures made from assembled and epitaxially connected colloidal nanocrystals (NCs) hold promise for crystalline solids with atomic and nanoscale periodicity, whereby the band structure can be tuned by the geometry. The formation of especially the honeycomb superstructure on a liquid substrate is far from understood and suffers from …
In an electron dot diagram of ethylene how many double bonds are present
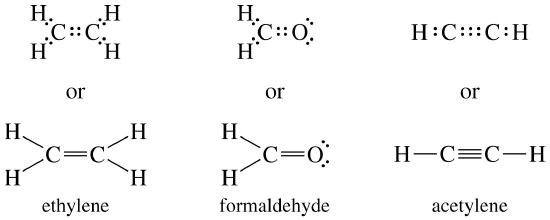
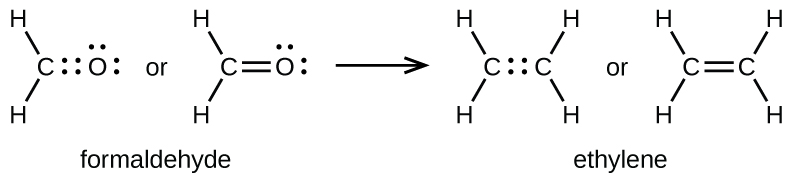
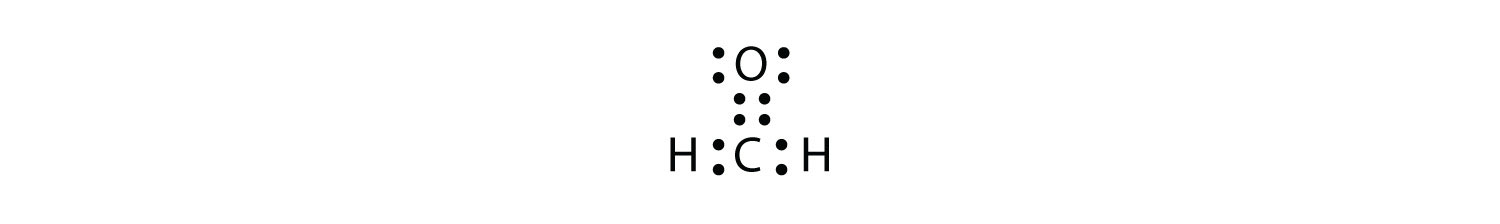
Since two electron pairs are shared there is a double bond between the two oxygen atoms. O2 Molecule with Double Covalent bond Ethylene Molecule: In ethylene, each carbon atom shares two of its valence electron with two hydrogen atoms and remaining two electrons with the other carbon atom. So there is a double bond between the carbon atoms. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. No lone pair is present on the central or outer atom in the lewis structure of C2H4. The lewis dot structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure. 1. Count total valence electron in C2H4 The left diagram shows a Lewis dot structure of sodium with ... We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in ...
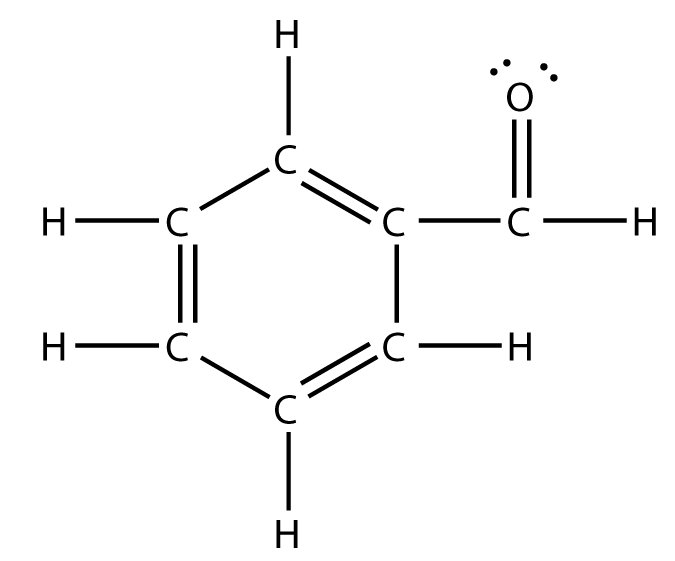
In an electron dot diagram of ethylene how many double bonds are present. A simple view of double covalent bonds. A double covalent bond is where two pairs of electrons are shared between the atoms rather than just one pair. Some simple molecules containing double bonds. Oxygen, O 2. Two oxygen atoms can both achieve stable structures by sharing two pairs of electrons as in the diagram. Chemists frequently use Lewis electron dot diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows: ... When it is warmer, the liquid phase of H 2 O is present. ... bond to an oxygen. If the atom is the same, double bonds have a higher priority than single ... In an electron dot diagram of ethylene (C2H4) how many double bonds are present?... What is the molecular shape of silicon tetrabromide? tetrahedron. Which of the following intermolecular forces plays a pivotal role in the unique properties of water? hydrogen-bonding. C2H4, or ethylene has a double bond between the two carbon atoms. The hydrogen atoms are singly bonded at an angle of 121 degrees from the carbon bonding.
By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later. The next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell.Its electron dot diagram resembles that of hydrogen, except ... Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms. The carbon atoms in ethene exhibit a valency of 4; therefore, in order to complete their outermost shell, the carbon atoms form a double bond with one another. Thus, the number of double bonds present in a molecule of ethylene is 1. Answer from: dbrwnn SHOW ANSWER 3 CHEM 1411. Chapter 7. Chemical Bonding I (homework) W e. AlBr 3 ____ 13. A chemical bond formed by two atoms sharing one or more pairs of electrons is called a(n) ____ bond. Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms. The carbon atoms in ethene exhibit a valency of 4; therefore, in order to complete their outermost shell, the carbon atoms form a double bond with one another. Thus, the number of double bonds present in a molecule of ethylene is 1. Advertisement Answer 4.8 /5 20 Megadeth
#Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Now how Lewis ... Electron dot structure of C2H4⟶H2C=CH2. solution. expand. Solve any question of Chemical Bonding and Molecular Structure with:- ... Academia.edu is a platform for academics to share research papers. Hydrogen bonds can be found between molecules of which substance? NH3. Which compound contains a triple bond? acetylene (C2H2) In an electron dot diagram of ethylene (C2H4), how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron.
If an atom lacks an octet, use electron pairs on an adjacent atom to form a double or triple bond. • Example: All the atoms have octets in this Lewis structure. Table 1.4 How to Write Lewis Structures.... H C O N O H H..:..
General-Chemistry-1.pdf - Free ebook download as PDF File (.pdf), Text File (.txt) or view presentation slides online.
Explanation: Lewis-dot structure: It shows the bonding between the atoms of a molecule as shown in the structure and it also shows the unpaired electrons present in the molecule. In the Lewis-dot structure the valence electrons are represented by 'dot'. The given molecule is propylene, C H 2 C H C H 3. . As we know that carbon has four valence ...
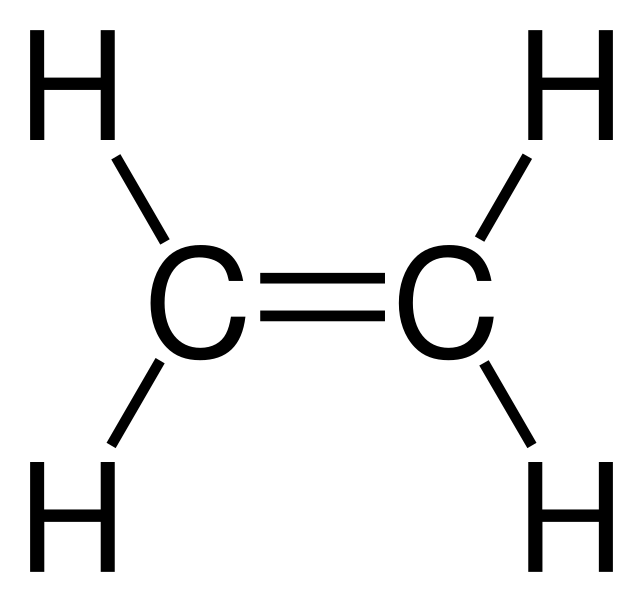
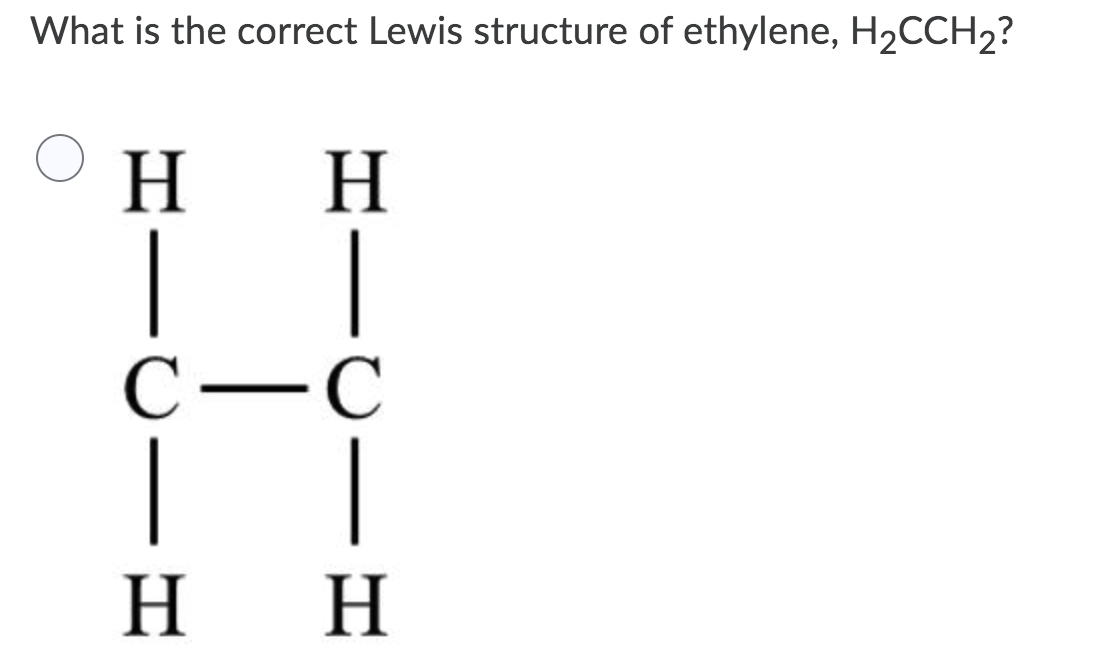
Since there are two bonds forming here, we will have a double bond structure. Hence, C2H4 is an alkene. Here, we have got the most suitable and appropriate Lewis Structure Sketch of ethylene. Molecular Geometry When we draw the Lewis Structure of C2H4, we find a linear 2-D representation. In reality, the molecular shape of ethene is not linear.
In an electron dot diagram of ethylene (C2H4) , how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron. Which intermolecular force plays a pivotal role in the unique properties of water? hydrogen bonding. Which element does not form a monatomic ion?
Academia.edu is a platform for academics to share research papers.
Oct 13, 2021 · How many electron domains does C2H4? An easy way to look at the Hybridization of Ethylene is to consider the electron domains on each Carbon atom. Since the double bond is considered a single domain, there will be three domains accounting for the covalent bonds with Hydrogen. Is C2H4 a double bond? Ethylene is a chemical compound with the ...
In contrast, Z-scheme faced many problems with electron mediators like having a complex fabrication process, high backward reaction, and shielding outcome by shuttle redox mediator. The p-n heterojunction WO 3 /BiOBr photocatalyst was synthesized by growing thin nanosheets of BiOBr on 1D WO 3 nanotubes bundles via a solvothermal process ( Ling and Dai, 2020 ).
20.12.2021 · The cover art illustrates the molecular structure of the first iron-carbene complex with a square-planar coordination environment. This geometry induces an unprecedented intermediate-spin configuration for the iron-carbene unit. The orbitally near-degenerate paramagnetic ground state exhibits large unquenched orbital momentum, manifesting in …
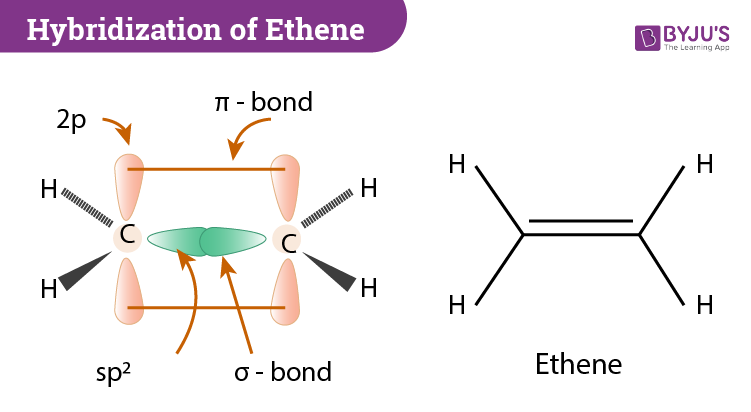
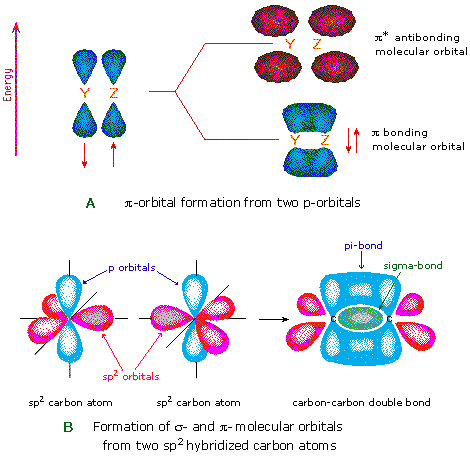
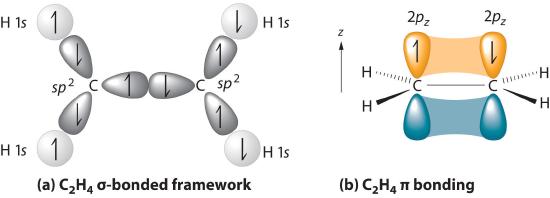
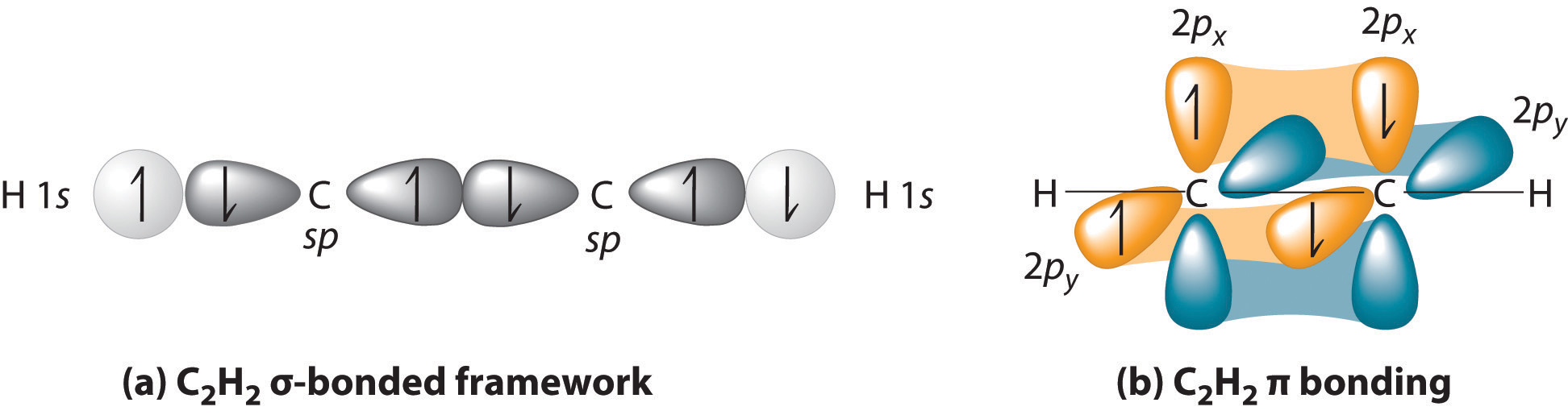
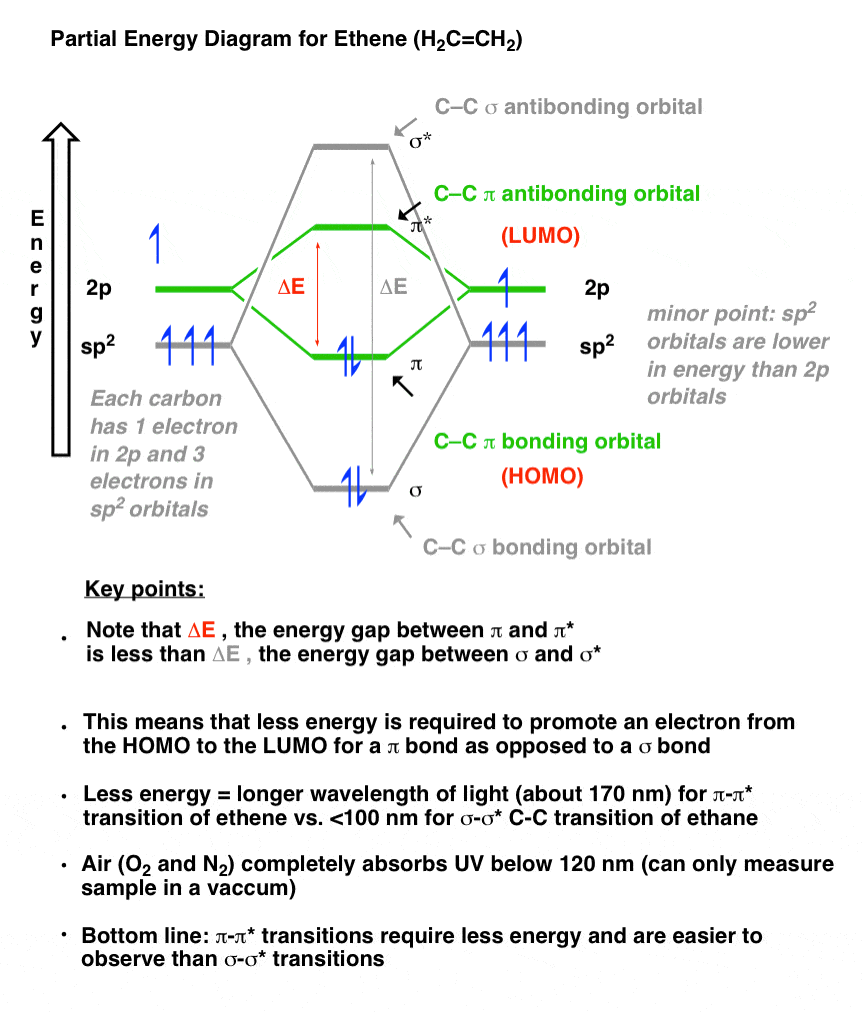
28.09.2021 ... Overall, ethylene is said to contain five sigma bonds and one pi bond. Pi bonds tend to be weaker than sigma bonds because the side-by-side ...
The number of covalent bonds in ethylene is: A 2 double bonds, 2 single bonds B 2 double and 4 single bonds C 2 single and 2 double bonds D 1 double and 5 single bonds Medium Solution Verified by Toppr Correct option is D 1 double and 5 single bonds Ethene means C2H4 and it is an alkene.
How to draw double and triple bonds using dots to represent valence electrons. ... and that is the correct dot structure for ethene or ethylene.
C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
Formation of ethyne (C2 H2) molecule In ethyne each of two carbon atoms share one electron with a hydrogen atom and 3 electrons between themselves forming triple bond between the two carbon atom The Lewis dot structure is Lewis representation of. C2H4 Molecular Geometry / Shape and Bond Angles C2H4 Molecular Geometry / Shape and Bond Angles
The nine sigma bonds consist of four between the four C ≡ N groups, and four between the C − C bond attached to cyanide and one sigma bond of ethylene, whereas there are nine pi bonds. Two pi bonds lie between each C ≡ N (cyanide) and therefore it will be eight pi bonds and one bond lies between C = C bond. So the total number of pi bonds is nine.
Bonding in Ethene. A key component of using Valence Bond Theory correctly is being able to use the Lewis dot diagram correctly. Ethene has a double bond ...
In an electron dot diagram of ethylene (C2H4), how many double bonds are present? One. What is the molecular shape of silicon tetrabromide? Tetrahedron. Which of the following intermolecular forces plays a pivotal role in biological molecules such as proteins and DNA? Hydrogen bonding.
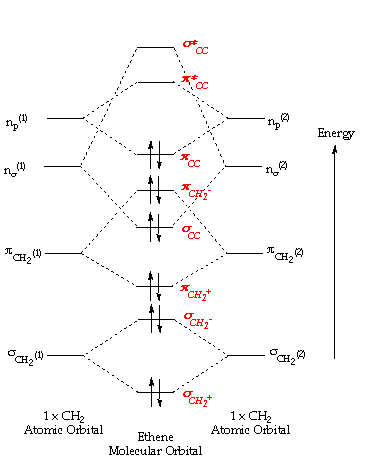
The bond consists of two electron clouds which lie above and below the plane of carbon and hydrogen atoms. (a) Formation of ethylene (b) Molecular orbital structure molecule of ethylene Thus, ethylene molecule consists of four sigma C - H bonds, one sigma C - C bond and one bond between carbon-carbon atom. The bond length of carbon-carbon ...
Drawing the Lewis Structure for C2H For C2H4 you have a total of 12 total valence electrons. Drawing the Lewis structure for C2H4 (named ethene) requires the use of a double bond. In a double bond two pairs of valence electrons are shared (for a total of four valence electrons).
The left diagram shows a Lewis dot structure of sodium with ... We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in ...
C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. No lone pair is present on the central or outer atom in the lewis structure of C2H4. The lewis dot structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure. 1. Count total valence electron in C2H4
Since two electron pairs are shared there is a double bond between the two oxygen atoms. O2 Molecule with Double Covalent bond Ethylene Molecule: In ethylene, each carbon atom shares two of its valence electron with two hydrogen atoms and remaining two electrons with the other carbon atom. So there is a double bond between the carbon atoms.



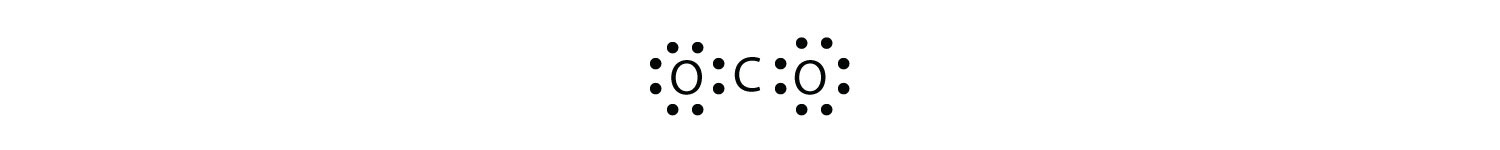















0 Response to "38 in an electron dot diagram of ethylene how many double bonds are present"
Post a Comment