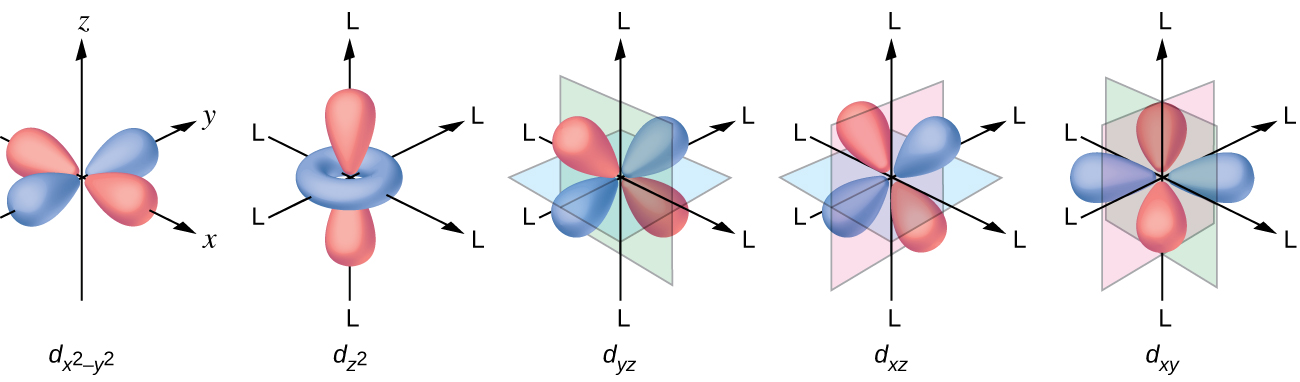
38 (a) Construct The D-orbital Energy Diagram Of An Octahedral Complex, W(co)6.
What is the crystal field splitting of an octahedral complex? - Quora For octahedral complex , there is six ligands attached to central metal ion , we understand it by following diagram of d orbitals in xyz plane. All the subshell of d orbital are not of same energy but we took an average and consider it as barricentere Those accquire lower energy gel depressed... SOLVED:Sketch the d -orbital energy level diagram for a typical... So typically for a cathedral conflicts is will fly against and setting instead of essentially having five do or bubbles with degenerate energy. The diagram will differ for tetra federal square planner and linear complexes, which may have reverted or may have less orbital as a result of just using S or P or...
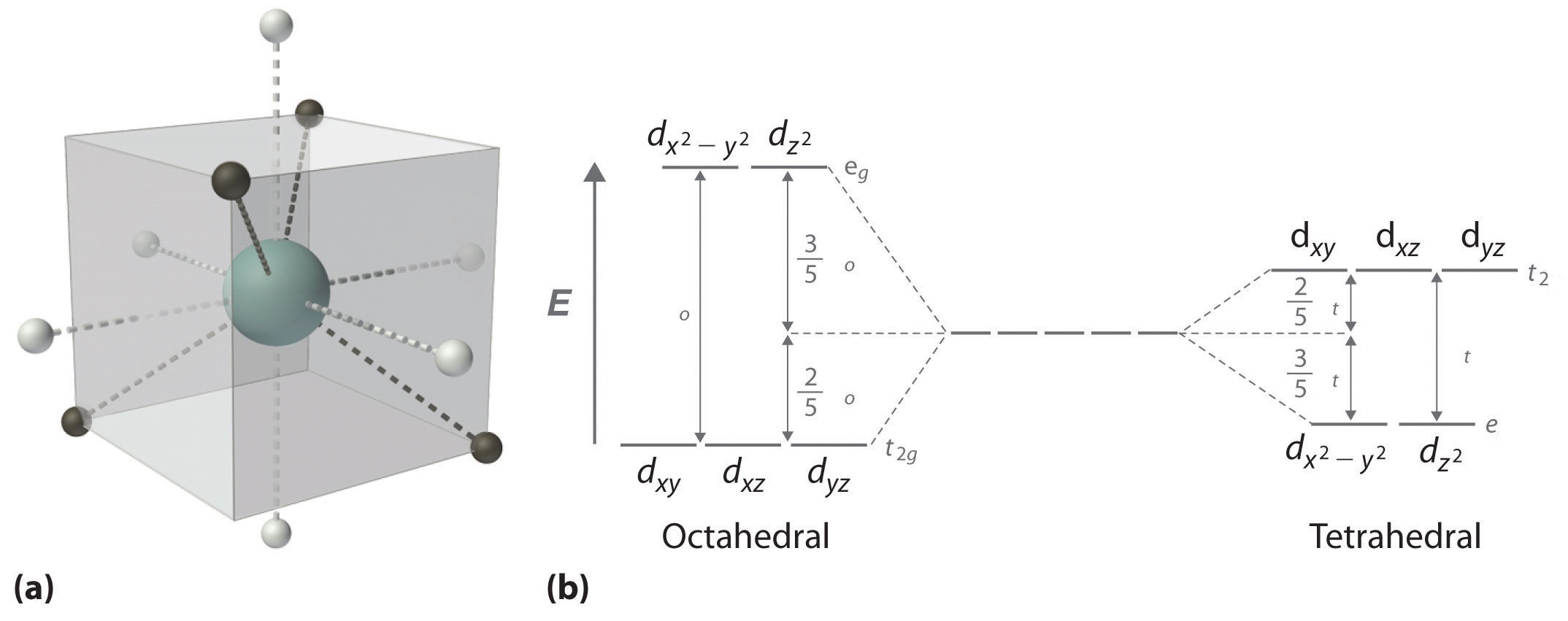
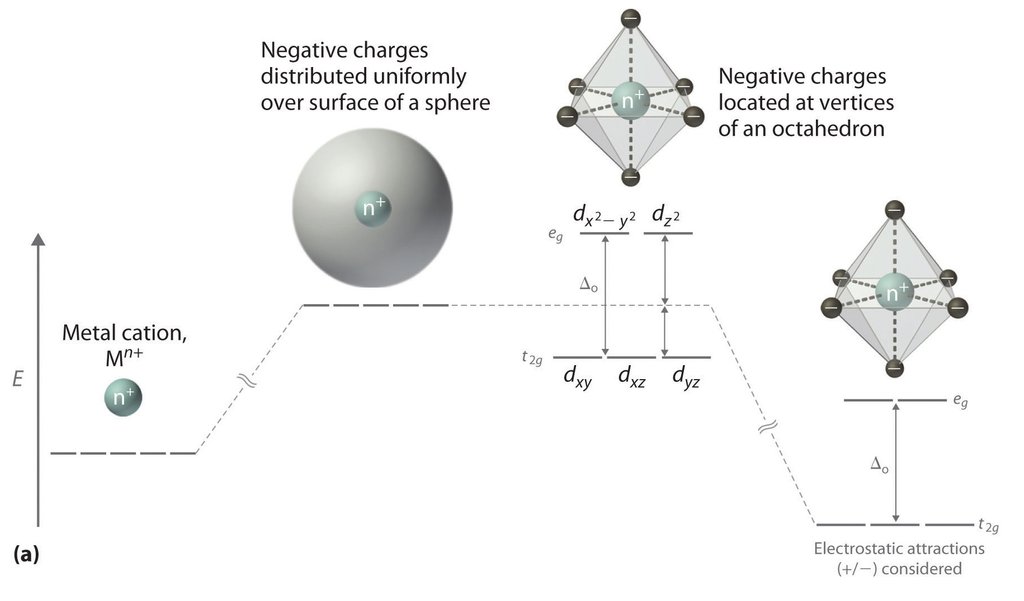
Crystal field theory - Wikipedia The most common type of complex is octahedral, in which six ligands form the vertices of an octahedron around the metal ion. Tetrahedral complexes are the second most common type; here four ligands form a tetrahedron around the metal ion. Octahedral crystal field stabilization energy.

(a) construct the d-orbital energy diagram of an octahedral complex, w(co)6.
Figure 3. Schematic diagram of the d-orbital splitting in octahedral... Download scientific diagram | Schematic diagram of the d-orbital 7(b) depicts the configurational coordinate diagram explaining the splitting of energy levels in the octahedral symmetry of Mn 4+ ions and the Generally, Mn 4+ ions can only generate luminescence in an octahedral or even above The influence of different co‐dopants on the luminescent characteristics has been elucidated in detail. Show by means of a diagram how the pattern of d orbital splitting... ...pattern of d orbital splitting changes as an octahedral complex undergoes tetragonal distortion and eventually becomes a square planar complex. (atomic number of Cr=24) had a magnetic moment of 3.83 B.M. The correct distribution of $$3d$$electrons in the chromium present in the complex is Orbital energy diagram complexes - Big Chemical Encyclopedia 1. Schematic orbital energy diagram representing various types of electronic transitions in octahedral complexes. A line connects an atomic orbital 10 Molecular orbital energy diagram of complexes 2, 18, and 20 compared to that of a Ti02 nanoparticle model. HOMO-LUMO gaps (eV) and lowest...
(a) construct the d-orbital energy diagram of an octahedral complex, w(co)6.. Crystal Field Theory | Octahedral Complexes d-Orbital Splittings. CFT focuses on the interaction of the five (n − 1)d orbitals with ligands arranged in a According to CFT, an octahedral metal complex forms because of the electrostatic interaction of a positively charged metal ion We can use the d-orbital energy-level diagram in Figure 23.10 "An... The d orbital electron configuration of octahedral complexes can... Draw a d-orbital splitting diagram for this complex and fill it with the appropriate number of electrons. Where does the final electron go in this diagram? Since the occupation of the eg orbitals in both complexes is the same it then follows that that the difference in the bond lengths must be due to the... inorganic chemistry - Which d-orbital configurations form an... With reference to crystal field splitting in octahedral complexes, my textbook shows the following table of distribution of $d$-orbital electrons in octahedral complexes, based on the energy difference Do ALL the $d$ orbital configurations (i.e. pertaining to all examples given) form octahedral complexes? Картинки по запросу "(a) construct the d-orbital energy diagram of an octahedral complex, w(co)6." Картинки по запросу "(a) construct the d-orbital energy diagram of an octahedral complex, w(co)6."
Draw a qualitatively energy-level diagram showing d-orbital splitting... (A) r-orbital splitting in the octahedral environment (D) The complex exists as lemon yellow crystals. (In the complex all electrons in t2g are paired and requires high radiation energy for excitation.) Crystal Field Theory - Octahedral Complex - Chemistry 2: Energy, energy level diagram for octahedral field complex crystal field theory properties of transition such of orbitals metal that hybridisation considers field theory. an octahedral. field. splitting. diagram. ←bary. centre. ( energy of. du orbital without. crystal field. Molecular orbital theory for octahedral complexes | Diagram simplified Molecular orbital theory Octahedral complexesDiagrams with examplesExplainedThanks for watching!!!!!#chemistry. Solved Construct the d-orbital energy diagram of octahedral Transcribed image text: Construct the d-orbital energy diagram of octahedral complex [CoCl_6]^4-. How many unpaired electrons are there in [CoCl_6]^4-? Calculate the Ligand Field Stabilization Energy (LFSE) of this octahedral complex with respect to delta_0.
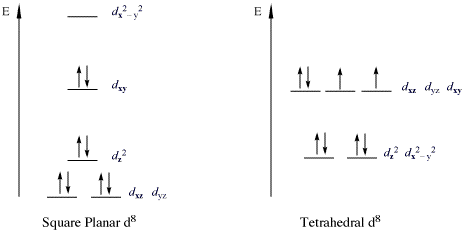
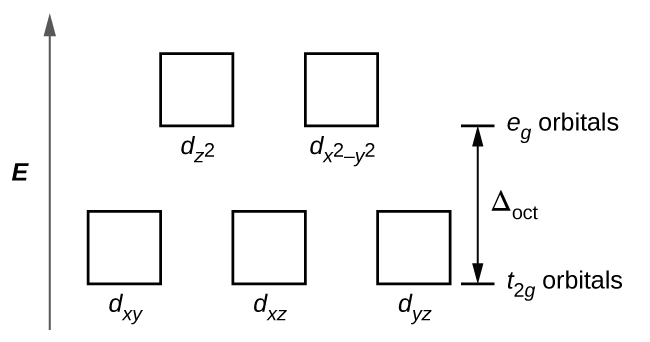
Introduction to Inorganic Chemistry/Coordination Chemistry and Crystal... Crystal field energy diagram for the d1 octahedral complex [Ti(H2O)6]3+. Referring to the molecular orbital diagram above, we see that the splitting between d-electron levels reflects Thus the energy difference between the t2g and eg orbitals can range between the energy of a rather... Bonding in Coordination Compounds: Crystal Field Theory | Boundless... Many reactions of octahedral transition metal complexes occur in water. For example, [Co(NH3)5Cl]2+ slowly aquates to give [Co CFT energy diagram for square planar complexes: Notice how the dx2 - y2 orbital is unfilled. The removal of a pair of ligands from the z-axis of an... Answers To Assignment2-2009 | PDF | Coordination Complex 5. Construct a complete orbital energy diagram for a tetragonally-distorted 6-coordinate complex, in which the axial bonds are weakened. You may thing of this as an extreme tetragonal octahedral complex in which the axial bonds are weakened to the limit. Consider -bonding only. HW Solutions #5 - Chemistry LibreTexts Both chromium(III) complexes are octahedral with an electron configuration of Cr3+ : [Ar]3d3. Using these facts, an energy level diagram of d-orbitals can be constructed. According to Hund's Rule, the three unpaired electrons go to the lower energy d-orbital as shown in the figure below.
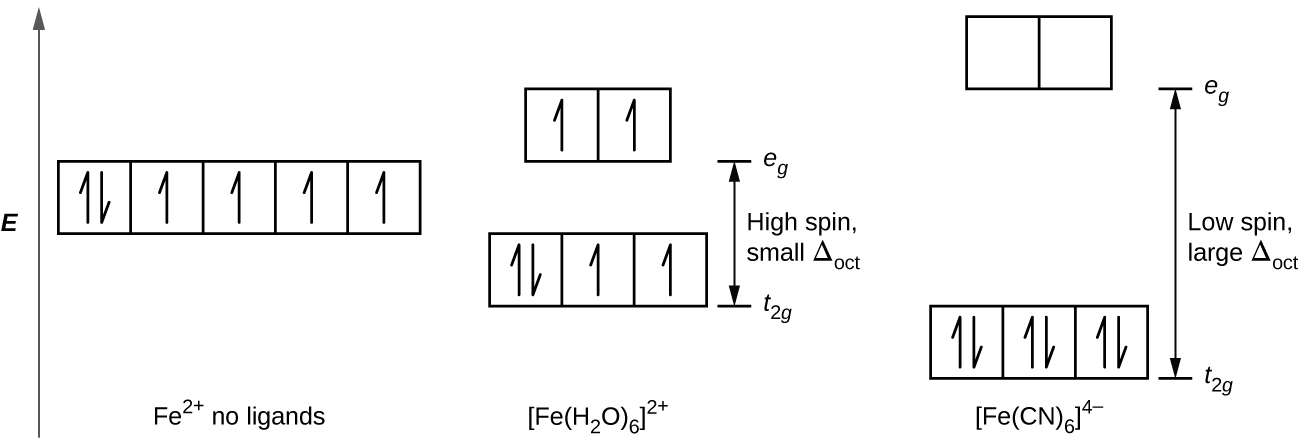
Splitting Of D-Orbital Energies In An Octahedral Fields - Coordination... Dec 16, 2021 - Splitting Of D-Orbital Energies In An Octahedral Fields - Coordination Chemistry Chemistry Notes | EduRev Example: [Co(CN)6]3- & [CoF6]3-. High Spin and Low Spin Complex. The energy difference between two sets of orbitals which arise due to an octahedral field is called ∆o...
Draw the crystal field energy level diagram for the... - Brainly.in An octahedral complex has one Metal ion with a charge of n+ on it. It is in the center, with four legands in Electron filling happens with each electron occupying a new orbital. This is due to the fact that pairing energy is more than splitting energy. (if the given complex is a strong electric field one...
PDF Microsoft Word - Questions and answers_Coordination.docx Tanabe‐Sugano diagrams are used in coordination chemistry to predict absorptions in the UV and 2) For a given multiplicity, the term with the largest value of L has the lowest energy, where L is the orbital angular 21. Why the magnetic moment of an octahedral monothiocarbamate complex of Iron (III)...
Answered: Draw the d-orbital splitting diagrams… | bartleby Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following: a. Zn2+ b. Co2+ c. Fe2+.
Crystal Field Theory Focus: energies of the d orbitals Assumptions... 3 _ ... _ Octahedral crystal field d orbital energy levels metal ion in octahedral complex Octahedral crystal field d orbital energy levels dz2 dx2- y2 _ ... _ E dxy dxz dyz isolated metal ion _ _ _ _ _ Metal ion and the nature of the ligand determines d-orbitals.
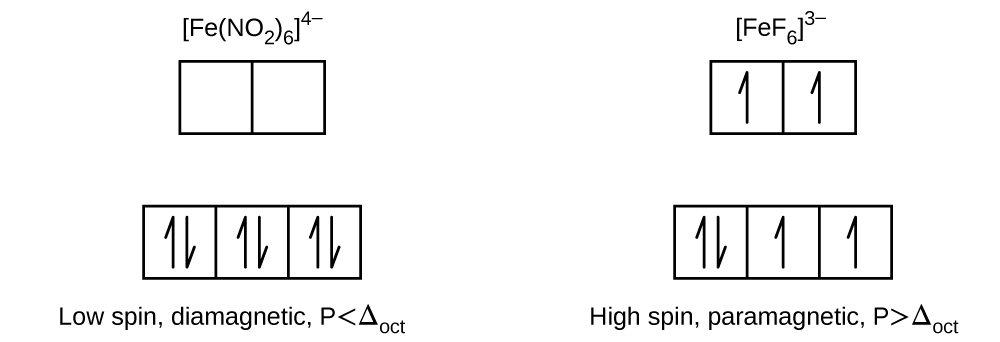
PDF Ch22Web.key | Sc Ti V Cr Mn Fe Co Ni Cu Zn d-orbital energy level diagrams octahedral complex. 5. Is the compound paramagnetic? This is a d6 low spin complex in an octahedral field. Three degenerate d orbitals are filled first, followed by the remaining two orbitals.
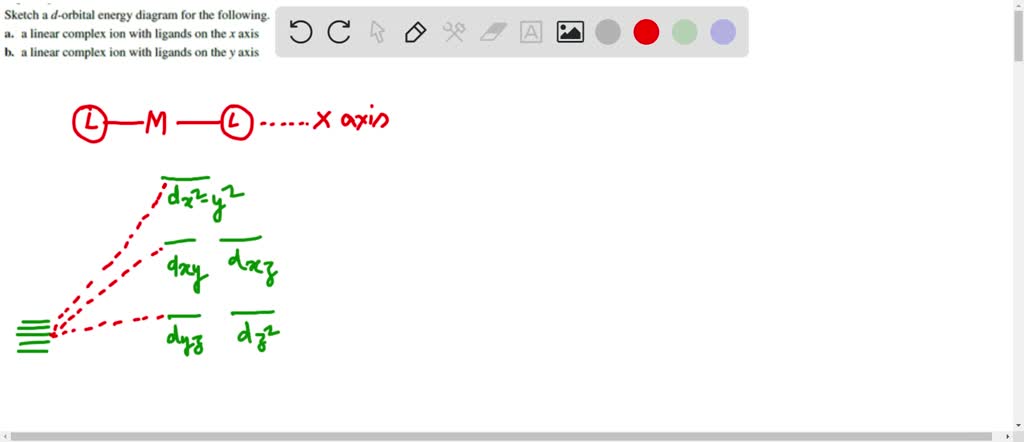
consider the pseudo octahedral complex ion of mathrmcr3 where a and mathrmb represent ligands ligand
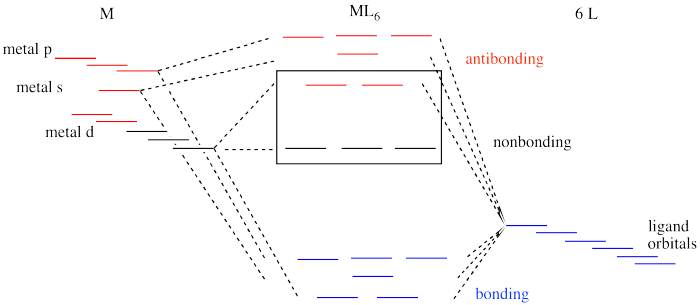
PDF Molecular Orbital Theory … Octahedral, Tetrahedral or Square Planar... In octahedral complexes, the molecular orbitals created by the coordination of metal center can be 6. The d z2 orbital of metal center can overlap with ligands atomic orbital approaching along x The molecular orbital energy level diagram for σ-bonding in square-planar complexes can be shown as
PDF Microsoft Word - webM-L and M-M bonding 2008-9.doc Construction and interpretation of octahedral ML6 molecular orbital energy diagram Lecture 3: π−acceptor ligands, synergic bonding, CO, CN Lecture 7: ML6 molecular orbital energy diagrams incorporating π−acceptor and π−donor ligands. Relationship to spectrochemical series, and the...
Lecture 7 - Crystal Field Theory for Octahedral Complexes The octahedral splitting energy is the energy difference between the t2g and eg orbitals. In an octahedral field, the t2g orbitals The free Fe3+ ion is a d5 ion. The two complexes are 6-coordinate and octahedral. First let's look at the d-orbital diagram for [Fe(H2O)6]3+: The first three electrons...
The crystal field splitting of an octahedral complex has... | Course Hero The higher energy levels aredz2and dx - y2, while the lower energy levels are dxy, dxz, and dyz. This diagram gives us values of 25 and 16. The second complex only has a single peak so the value cannotbe calculated, but if there were two peaks the value of B would be close to 1075. the...
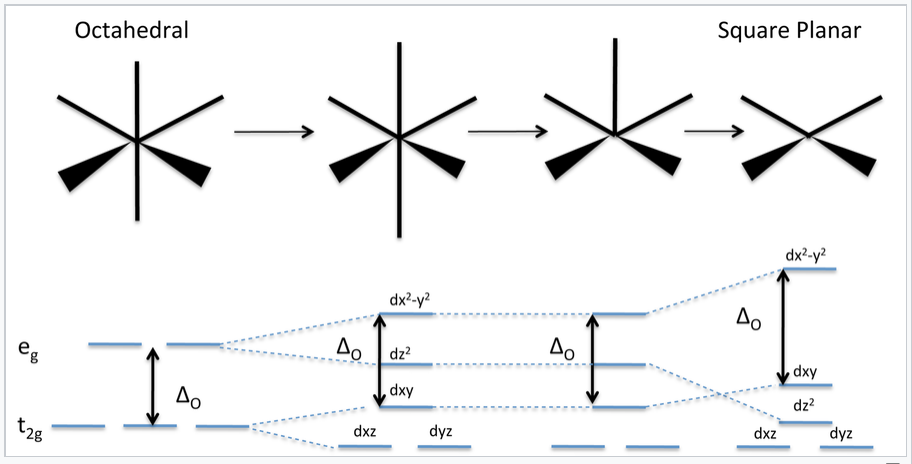
Molecular Orbital Theory (MOT), Bonding in [Fe(CN) 6] 4- ion - The... The MO energy level diagram for metal complexes are complicated than that of simple diatomic This is crystal field stabilisation energy, CFSE. It gives useful data for relative stability of a complex. 18. Draw the diagram to show the pattern of d orbital splitting changes in octahedral, tetragonal and...
Crystal Field Theory - Octahedral Complexes | Chemistry | JoVE In octahedral complexes, the eg orbitals are further destabilized (higher in energy) compared to the t2g orbitals because the ligands interact more strongly with the d orbitals at which they are pointed directly. In octahedral complexes, the lobes in two of the five d orbitals, the dx2−y2 and dz2 orbitals...
Orbital energy diagram complexes - Big Chemical Encyclopedia 1. Schematic orbital energy diagram representing various types of electronic transitions in octahedral complexes. A line connects an atomic orbital 10 Molecular orbital energy diagram of complexes 2, 18, and 20 compared to that of a Ti02 nanoparticle model. HOMO-LUMO gaps (eV) and lowest...
Show by means of a diagram how the pattern of d orbital splitting... ...pattern of d orbital splitting changes as an octahedral complex undergoes tetragonal distortion and eventually becomes a square planar complex. (atomic number of Cr=24) had a magnetic moment of 3.83 B.M. The correct distribution of $$3d$$electrons in the chromium present in the complex is
Figure 3. Schematic diagram of the d-orbital splitting in octahedral... Download scientific diagram | Schematic diagram of the d-orbital 7(b) depicts the configurational coordinate diagram explaining the splitting of energy levels in the octahedral symmetry of Mn 4+ ions and the Generally, Mn 4+ ions can only generate luminescence in an octahedral or even above The influence of different co‐dopants on the luminescent characteristics has been elucidated in detail.






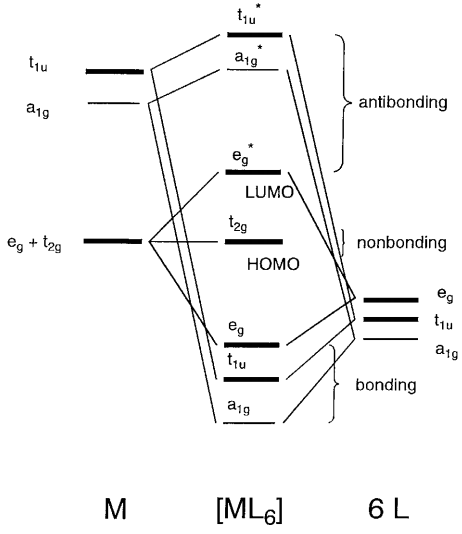



![Molecular orbital diagram of [CoF 6 ] 3-complex with six p ...](https://www.researchgate.net/profile/Majid-Monajjemi/publication/257140982/figure/tbl1/AS:669050661257229@1536525516648/Molecular-orbital-diagram-of-CoF-6-3-complex-with-six-p-donor-ligands.png)




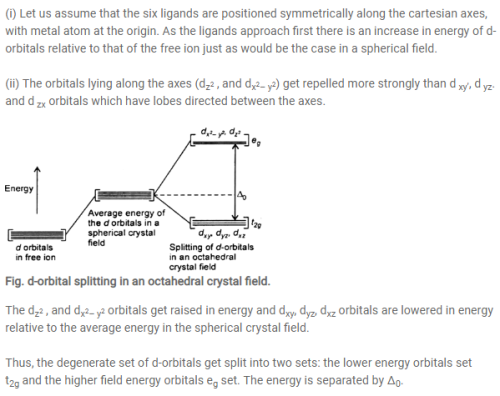

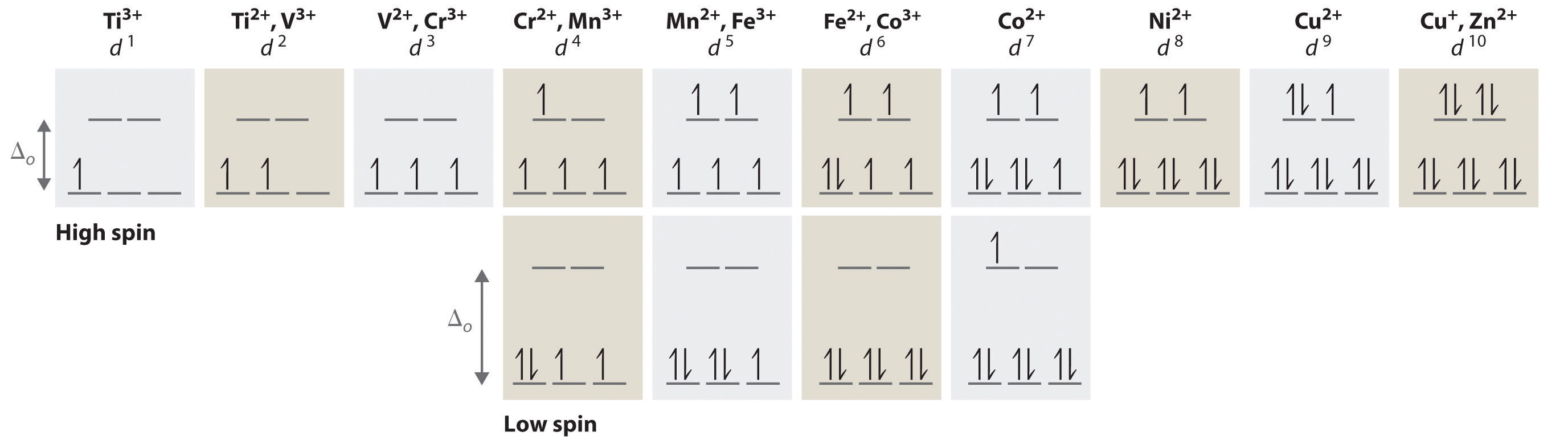
0 Response to "38 (a) Construct The D-orbital Energy Diagram Of An Octahedral Complex, W(co)6."
Post a Comment