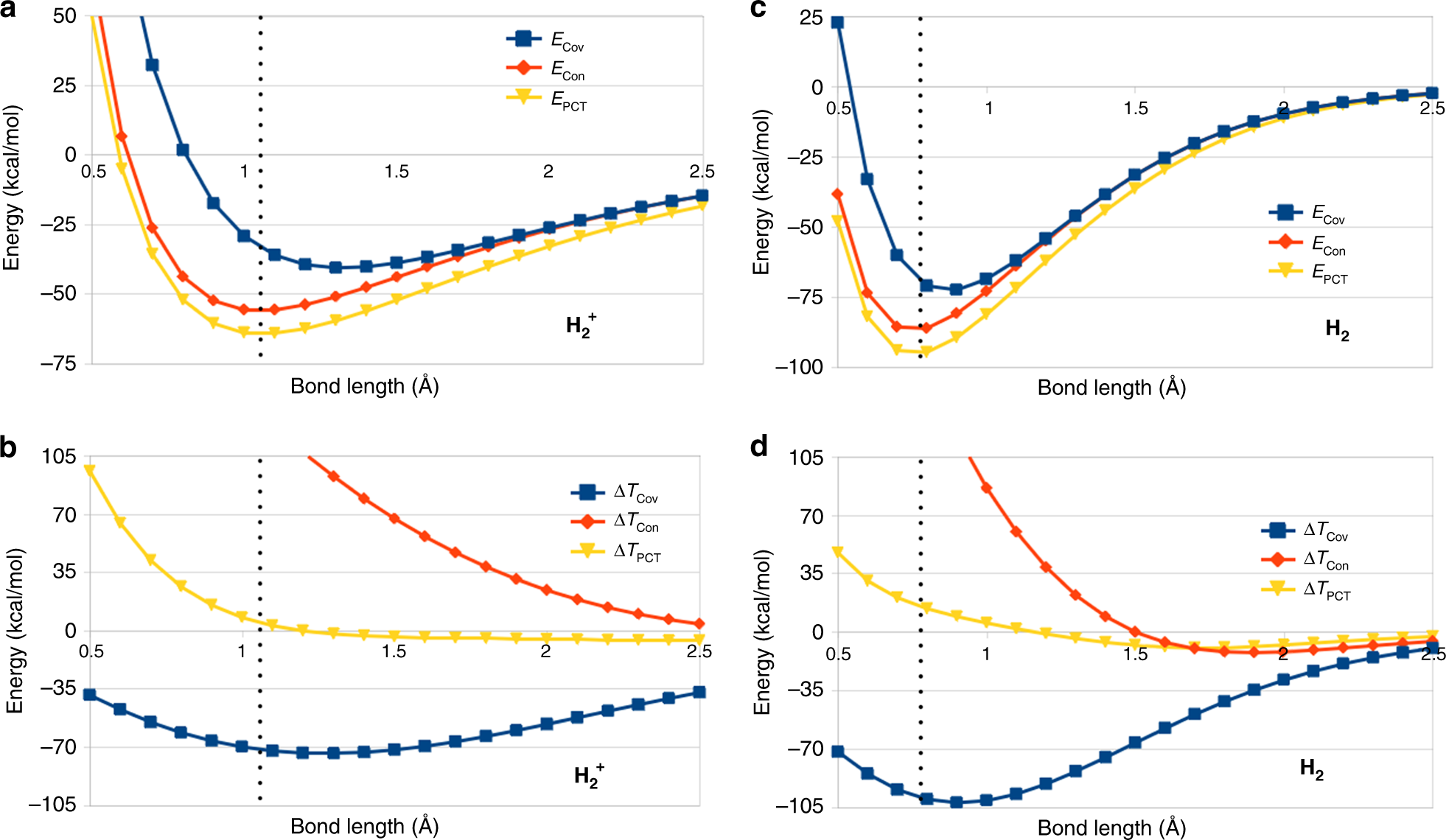
37 which type of chemical bond occurs when atoms share electrons, as shown in this diagram?
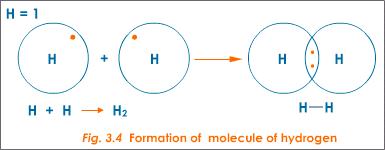
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds.The strength of chemical bonds varies considerably; there are "strong bonds" … In a single covalent bond, when the electrons are shared between two s orbitals, the resulting bond is a sigma (σ) bond as shown in Figure 3-4. Sigma bonds are the strongest covalent chemical bonds. Sigma bonds also occur when an s and a p orbital share a pair of electrons or when two p orbitals that are parallel to the internuclear axis share a pair of electrons (see …
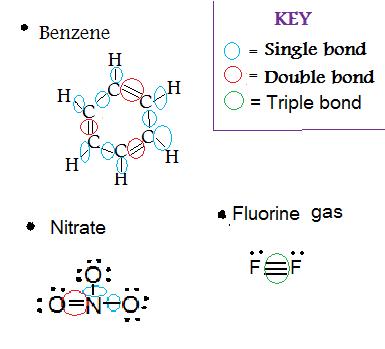
25-04-2019 · Due to the presence of one negative charge, number of valence electrons = 5 + 1 = 6. One O-atom forms two bonds (= bond) and two O-atom are shared with two electrons of N-atom. Thus, 3 O-atoms are shared with 8 electrons of N-atom. Number of bond pairs (or shared pairs) = 4 Number of lone pairs = 0. Q7.
Which type of chemical bond occurs when atoms share electrons, as shown in this diagram?
The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [Ne]3s 2 configuration, is analogous to its family member beryllium, [He]2s 2.Both atoms have a filled s subshell outside their filled inner shells. Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3s 2 3p 1, is analogous to its family member boron, [He]2s 2 2p 1. Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education research and engage students through an intuitive, game-like environment where students learn through exploration and discovery. An introduction to water and its structure. Local structures and water clusters . It is quite likely that over very small volumes, localized (H 2 O) n polymeric clusters may have a fleeting existence, and many theoretical calculations have been made showing that some combinations are more stable than others. While this might prolong their lifetimes, it does not appear that …
Which type of chemical bond occurs when atoms share electrons, as shown in this diagram?. 01-03-2016 · The removable electrons are found in a lower energy state, indicating higher energy consumption when they are promoted. The electron configurations of Mn 4+ and Ru 4+ are shown in Fig. 14: four electrons occupy the t 2g orbital with a high spin state in Ru 4+ and three electrons fill the t 2g orbital in Mn 4+. Academia.edu is a platform for academics to share research papers. An introduction to water and its structure. Local structures and water clusters . It is quite likely that over very small volumes, localized (H 2 O) n polymeric clusters may have a fleeting existence, and many theoretical calculations have been made showing that some combinations are more stable than others. While this might prolong their lifetimes, it does not appear that … Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education research and engage students through an intuitive, game-like environment where students learn through exploration and discovery.
The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [Ne]3s 2 configuration, is analogous to its family member beryllium, [He]2s 2.Both atoms have a filled s subshell outside their filled inner shells. Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3s 2 3p 1, is analogous to its family member boron, [He]2s 2 2p 1.
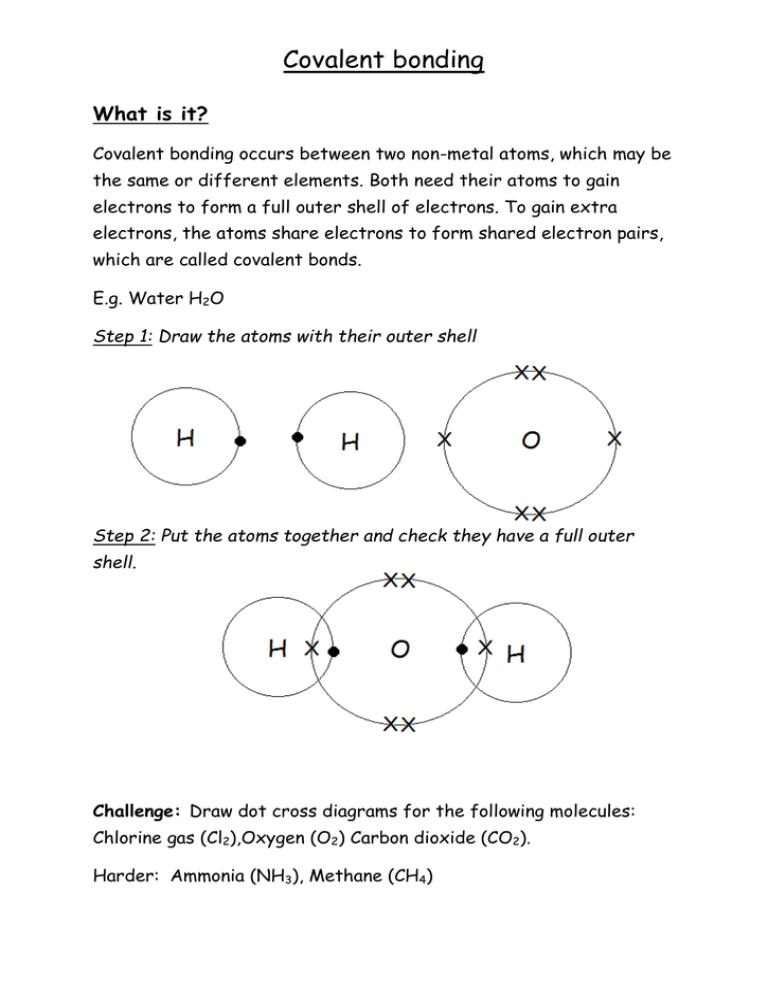


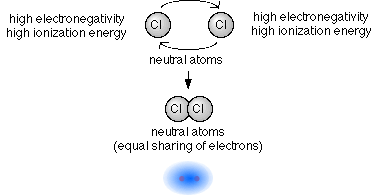




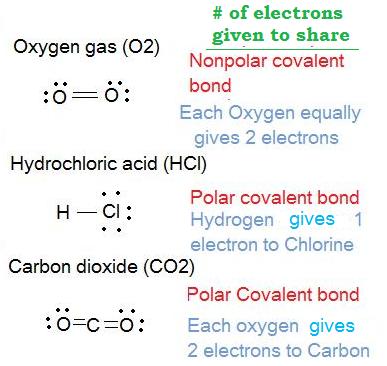




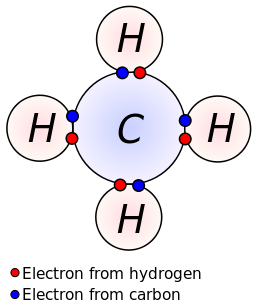
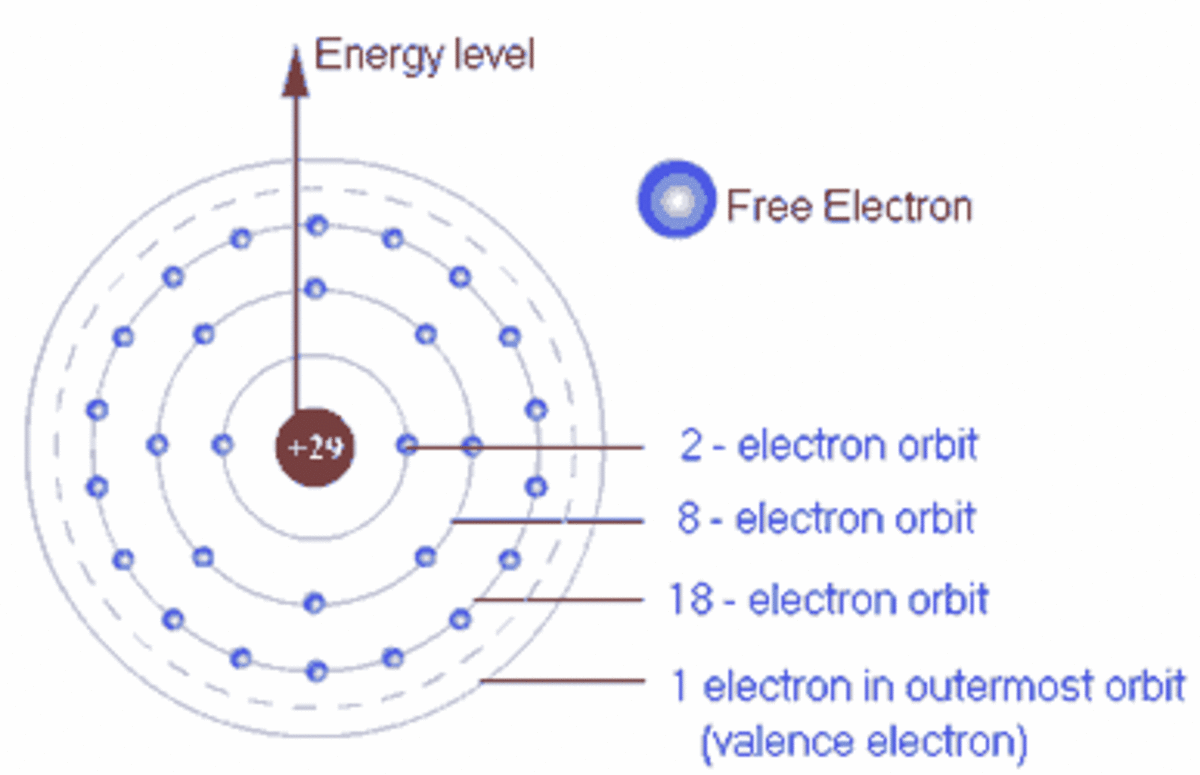
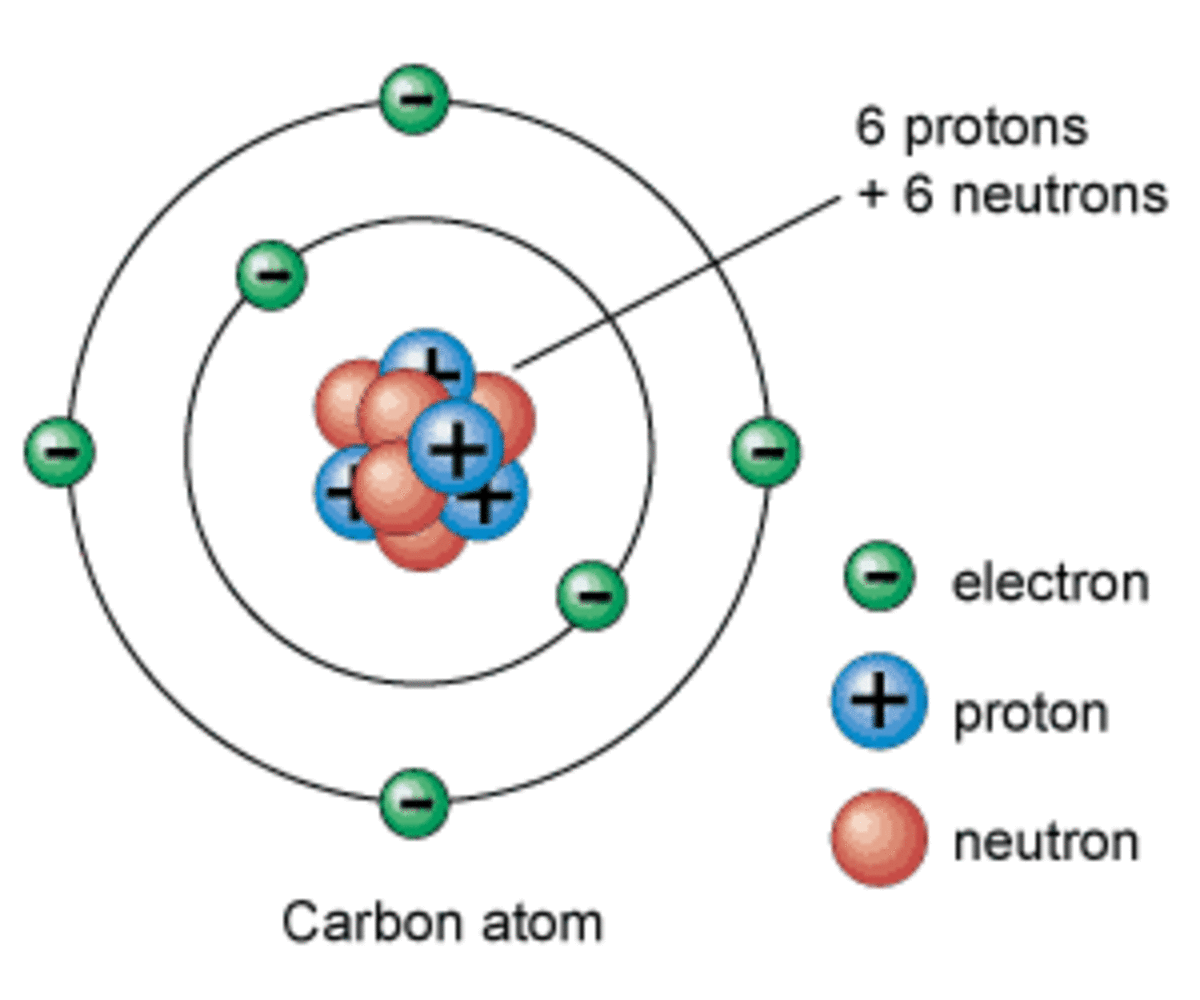
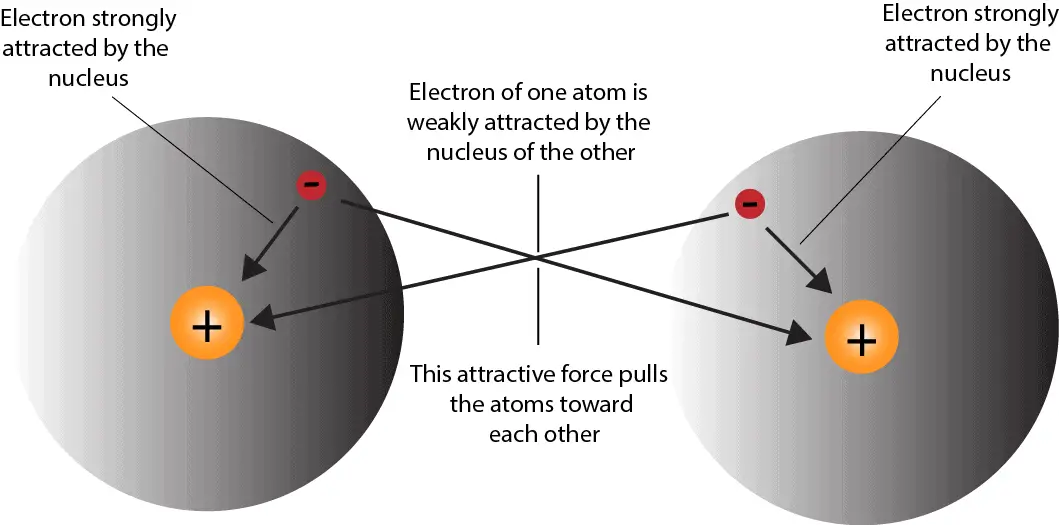








0 Response to "37 which type of chemical bond occurs when atoms share electrons, as shown in this diagram?"
Post a Comment