37 Cn- Mo Diagram
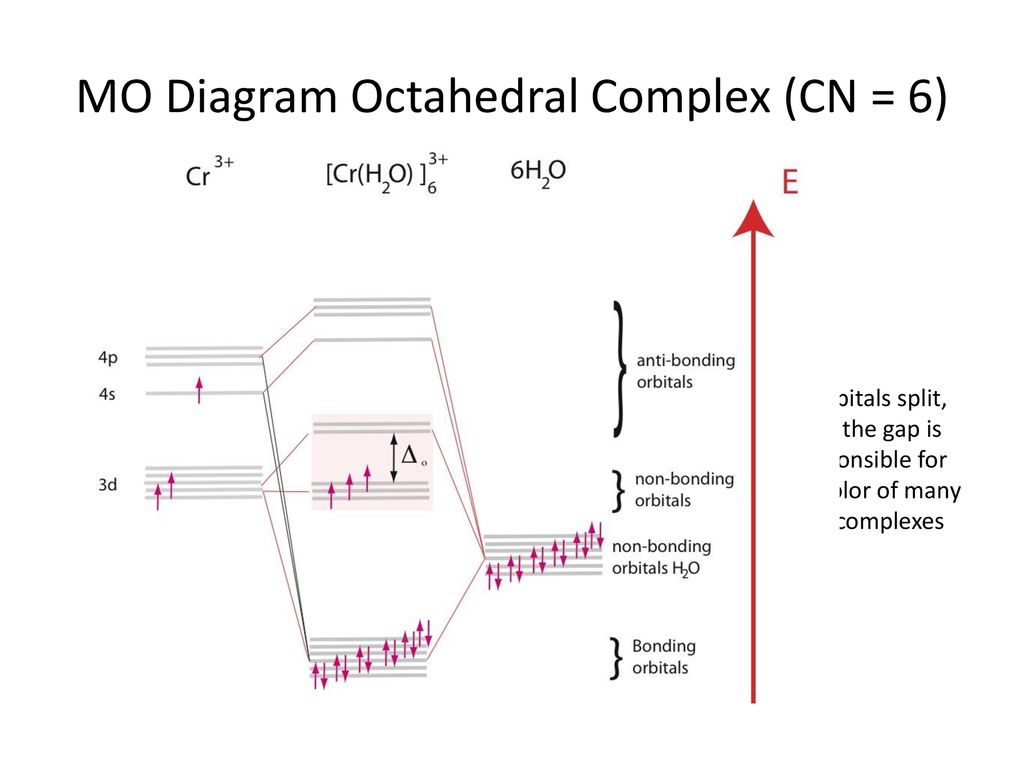
The correct energy level diagram for [Co(CN)6]^3 Draw energy level diagrams and indicate the occupancy of the orbitals in the following complexes : (a) d 6 octahedral, low-spin (b) d 9 octahedral with tetragonal elongation (c) d 8 square planar (d) d 6 tetrahedral. Calculate in units 0 the difference in crystal field stabilization energy between complexes (a) and (b) assuming that the ligands are strong field ligands. Molecular Orbitals for Carbon Monoxide - Newcastle University The Molecule. CO is a very stable 10-valence-electron molecule, isoelectronic with [CN] - and with N 2, which has a slightly lower bond dissociation energy than CO. The formal bond order of CO is 3, from about one σ- bond and two π- bonds. Its most important property is burning in air to give CO 2 , in the combustion of fossil fuels.
PDF SALCS for Common Geometries bonding) Example: Constructing a MO for Hexammine 2+Ruthenium, [[(Ru(NH 3) 6] NH 3 NH 3 2+ H 3N Ru NH 3 point group = O h H 3N NH 3 h 48 Ru bonding AOs O h E 8C 3 6C 2 6C 4 3C 2 i6S 4 8S 6 3 h 6 d /h 60 02 20004 2 = A 1g: 5s T 1u: (5p x , 5p y, 5p z) E g: (4dx2‐y2, 4dz2) A 1g 6001260001212481 A 2g 6 0 0 -12 6 0 0 0 12 -12 0 0 E g 1200012000240 48 1 Pd non‐bonding AOs
Cn- mo diagram
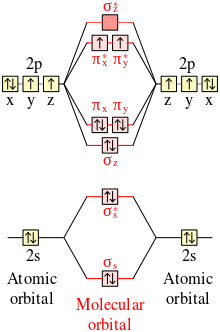
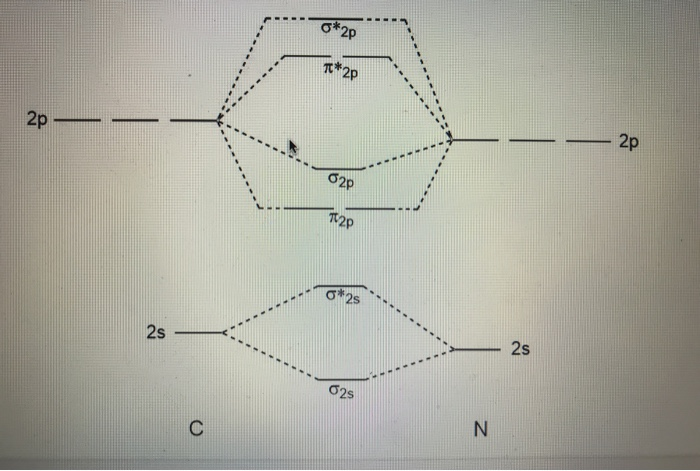
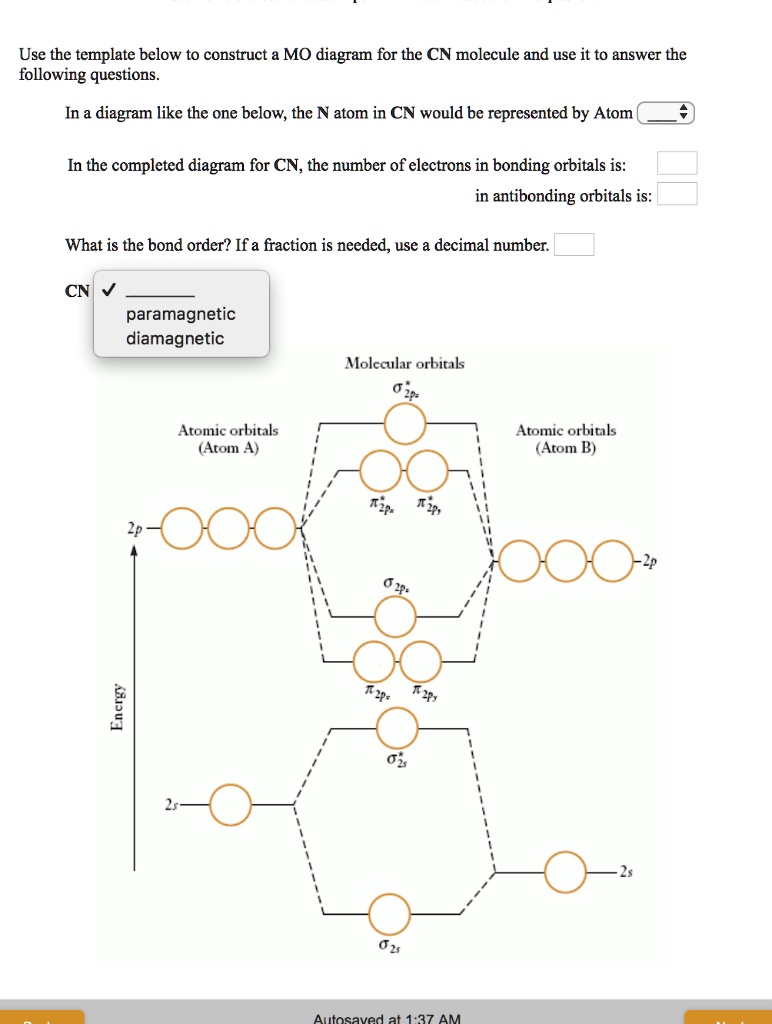
CN- lewis structure, molecular orbital diagram, and, bond order Also, using the Molecular orbital diagram of CN- we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. 7.3: How to Build Molecular Orbitals - Chemistry LibreTexts General Notes on Molecular Orbital Diagrams. The Y-axis of a MO diagram represents the total energy (not potential nor Gibbs Energy) of the orbitals. Individual atomic orbitals (AO) are arranged on the far left and far right of the diagram. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram. PDF Bonding in transition metal complexes Example: Constructing a MO diagram for Chromium Hexacarbonyl, Cr(CO) 6 Cr ππππ-bonding AOs T2g:(3dxy,3dxz,3dyz) T1u:(4px,4py,4pz) • T2g previouslyconsiderednon-bondingin σ-bondingscheme • T1ucombineswith T1u SALCin in σ-bondingscheme • T1g, T2u π-SALCs are non-bonding Cr non-bonding AOs T2g: (3 dxy, 3dxz, 3dyz) Cr σσσσ-bonding ...
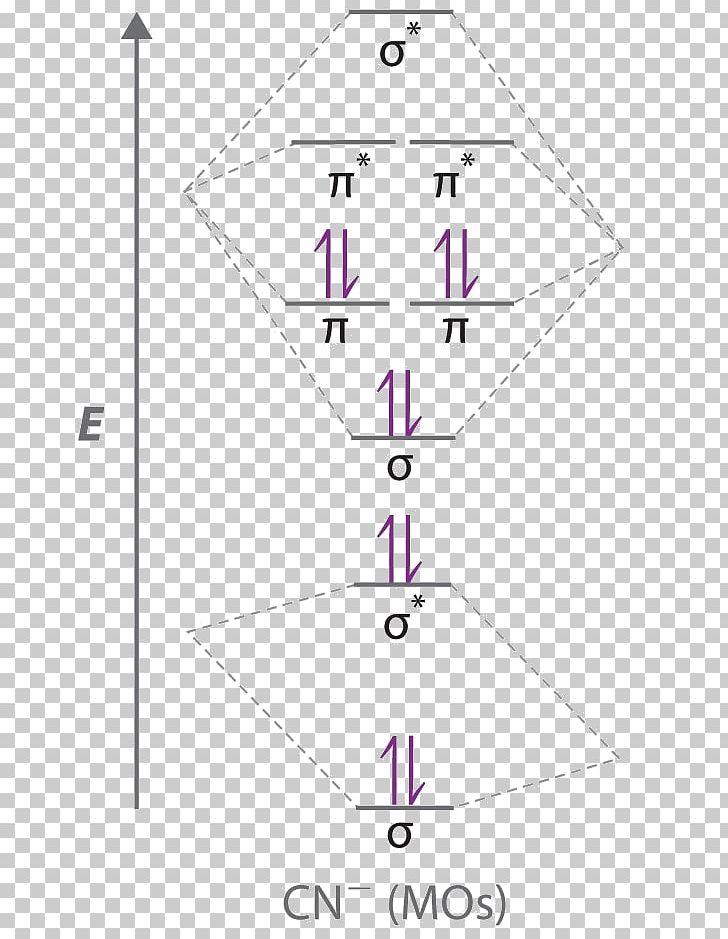
Cn- mo diagram. MO Diagram of CN-< - Hunt Research Group MO Diagram of CN-< Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis The process Write down as far as you can remember the steps that we will need to go through ... for example the first step will be to draw a set of axes ... answer PDF Lecture 6 4 coordinate complexes, summary, typical exam ... Walsh diagram OH 2, SH 2, NH 2 8 Bent-, FH2 + NH 2, PH 2, CH 2 7 Bent-, OH2 + CH 2, SiH 2, BH 2 6 Bent-, NH2 + BH 2, AlH 2, CH 2 5 Bent BeH 2, BH 2+ 4 Linear LiH 2, BeH 2+ 3 Linear LiH 2+ 2 Bent No. of Shape valence electrons Molecular species Known shape of some AH 2 molecules Recall: a molecule adopts the structure that best stabilises the HOMO. MO Diagram #3 - CN- - YouTube This video is about MO Diagram #3 - CN- Cyanide Molecular Orbital Diagram - schematron.org CN- (Cyanide ion), NO+ (Nitrosonium ion ). The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. We must remember that total number of electrons in carbon is six. What do the molecular orbitals of cyanide look like, compared with those paired HOMO's and LUMO's in the molecular orbital energy diagram.
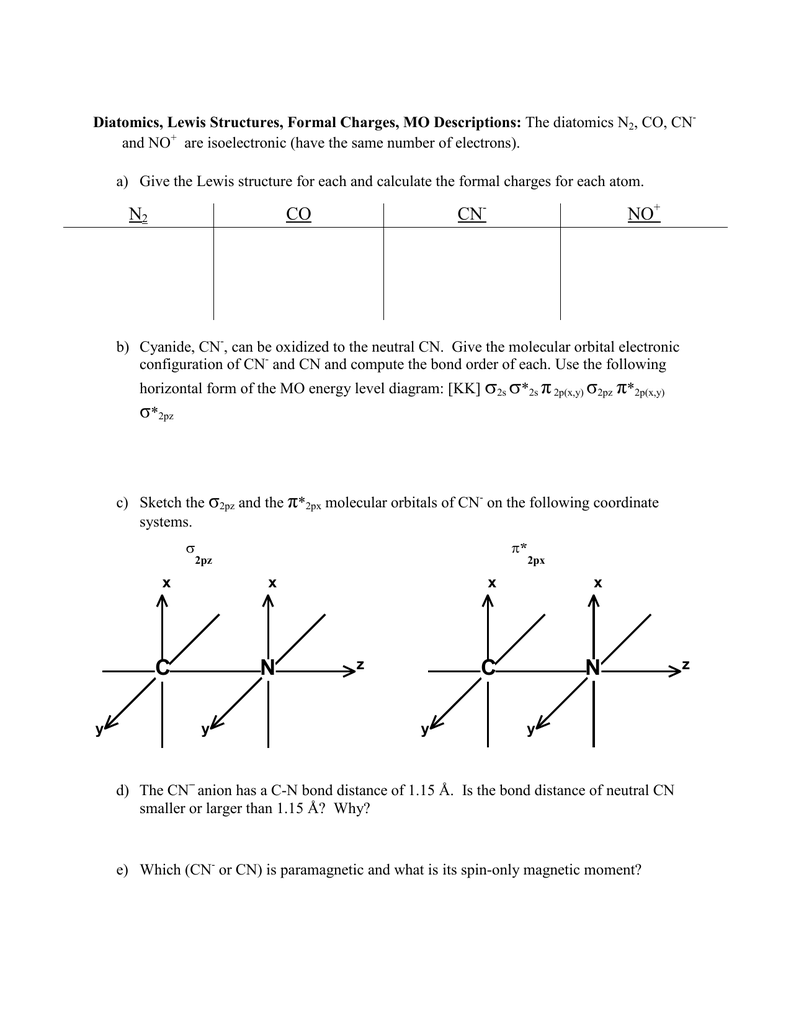
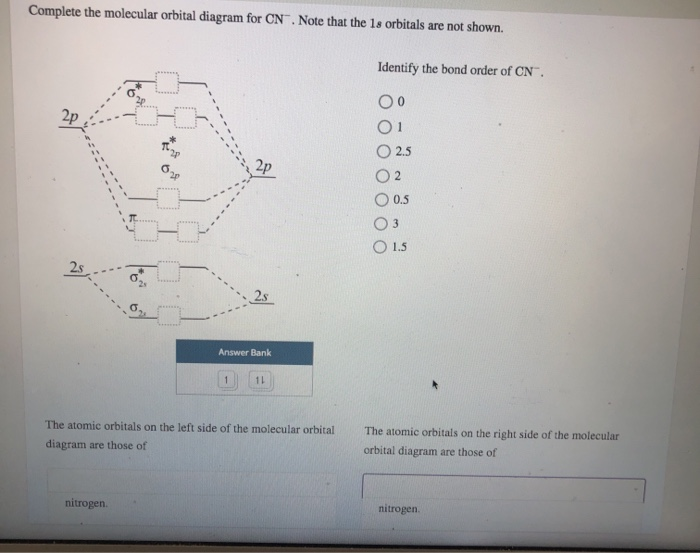
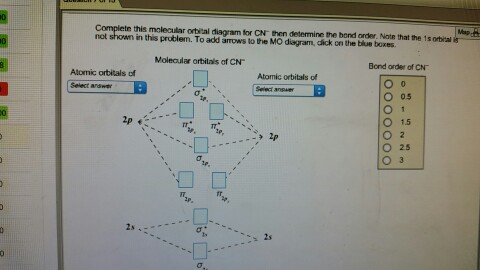
Molecular Orbital Theory Heteronuclear Diatomic (Cyanide ... Dr. Shields shows you how to draw the MO correlation diagram for cyanide (CN-), calculate the MO bond order, and write the MO electron configuration with an ... What is the bond order of CN-? - Quora Answer (1 of 6): \text{Bond order} = \frac{n_{\text{bonding electrons}}-n_{\text{antibonding electrons}}}{2} Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B.O.=\frac{8-2}{2}=3 Here's a guide on how to construct MO diagrams, i... The molecular orbital configuration of CN^+ is - Toppr Ask The molecular orbital configuration of C N + is K K σ (2 s) 2, σ ∗ (2 s) 2, π (2 p x ) 2, π (2 p y ) 2. Bond order is 2 . All the electrons are paired and ion is diamagnetic. Solved Complete the molecular orbital diagram for CN. Note ... Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of.
PDF MO Diagrams for More Complex Molecules MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection. Can you please describe the MO diagram of CN? Tania Havenga. University of South Africa. Here is the MO for CN-, just take away a single electron from the MO since CN is neutral. Vijayta Gupta is right, the N atom is lower in energy. http ... MO Diagram of CN-< - Hunt Research Group This means that very occasionally an occupied MO can move up in energy as long as the occupied MOs move down more in energy. If only two MOs mix then one always goes up and one down, and the one that goes up better be unoccupied. However, once a third MO starts mixing strongly the situation is extremely complex, and this is what has happened here. Solved: Compare the MO descriptions for CN, CN+, CN2+, CN ... 49CE. 50CE. 51BYK. 52BYK. 53BT. 54BT. 55BT. Compare the MO descriptions for CN, CN +, CN 2+, CN -, and CN 2-. Refer to the preceding diagram but assume that the π 2py and π 2pz MOs are lower in energy than the σ 2p MO.
Molecular nitrogen, carbon monoxide, and c ... - Clutch Prep Q. Draw Lewis structures and MO diagrams for CN+, CN, and CN-. According to the Lewis model, which species is most stable? Q. Use molecular orbital theory to complete this table. Q. What is the ground-state electron configuration of O2^-? 1. (δ2s)^2(δ2s*)^2(π2p)^3(π2p*)^1 2.
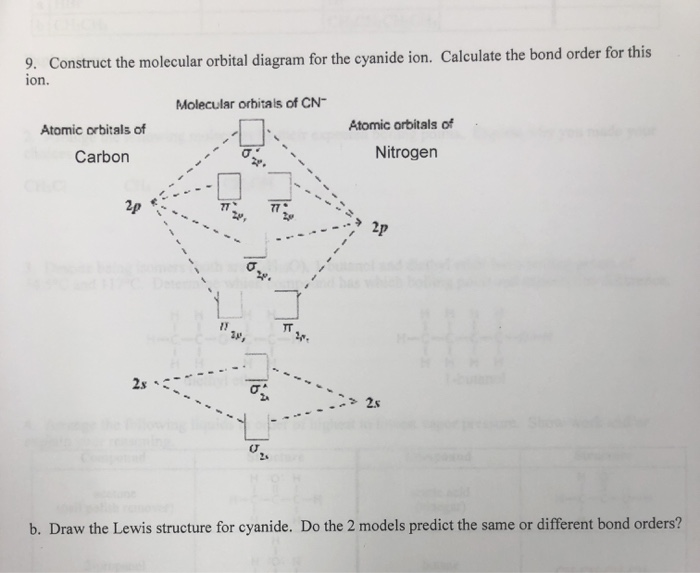
Complete this molecular orbital diagram fo... | Clutch Prep We're being asked to complete the molecular orbital diagram of CN-and then determine the bond order. To do so, we shall follow these steps: Step 1: Calculate the total valence electrons present. Step 2: Fill the molecular orbitals with electrons. Step 3: Determine the bond order. Step 1: Calculate the total valence electrons present. Group ...
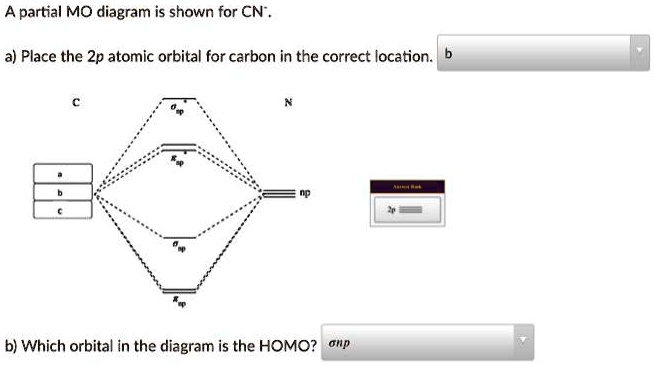
inorganic chemistry - How can one tell from the MO diagram ... The cyanide ion, when acting as a nucleophile, typically attacks via carbon as its HOMO has a greater contribution from carbon. But how can we deduce this from the MO diagram? To me it appears that the nitrogen $\mathrm{2p}$ orbitals are energetically closest to the $3\sigma$ HOMO, so its coefficient should be larger in the HOMO.
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Cyanide ion | CN- - PubChem Cyanide ion | CN- | CID 5975 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ...
42 complete this molecular orbital diagram for cn - Wiring ... Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. orbital from the σ-bonding.
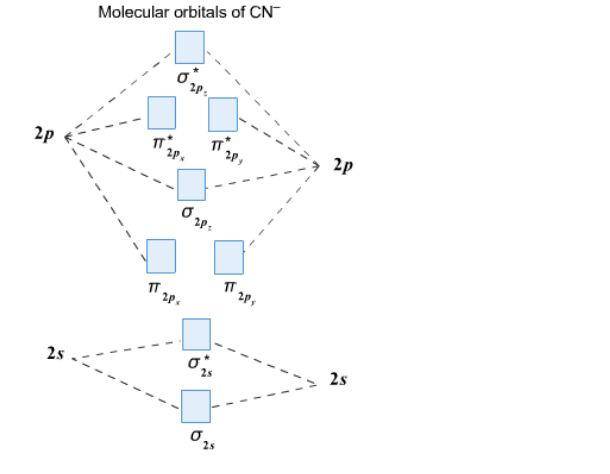
Cyanide Molecular Orbital Diagram - Wiring Diagrams The resul7ng MO diagram looks like this. CN- (Cyanide ion), NO+ (Nitrosonium ion). Figure \ (\PageIndex {2}\): Molecular Orbital Energy-Level Diagram for \ (\ PageIndex {12}\) to describe the bonding in the cyanide ion (CN −). In the traditional Lewis picture there are two lone pairs on the cyanide ion.
CN Lewis Structure, Molecular Geometry, Hybridization ... 2 days ago · CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
PDF Lecture 3 - chem.tamu.edu Lecture 3 Ligands and Bonding and Electron Counting in Organo-Transition Metal Compounds . Stable electronic configurations: MO Energy Level Diagrams Reviewed
MO Diagrams - GitHub Pages The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron. You can either draw the `O_2` diagram and remove `1` electron, or just draw the `O_2^+` diagram. The diagram will end up as such: Notice the effect that this has on the overall bonds.
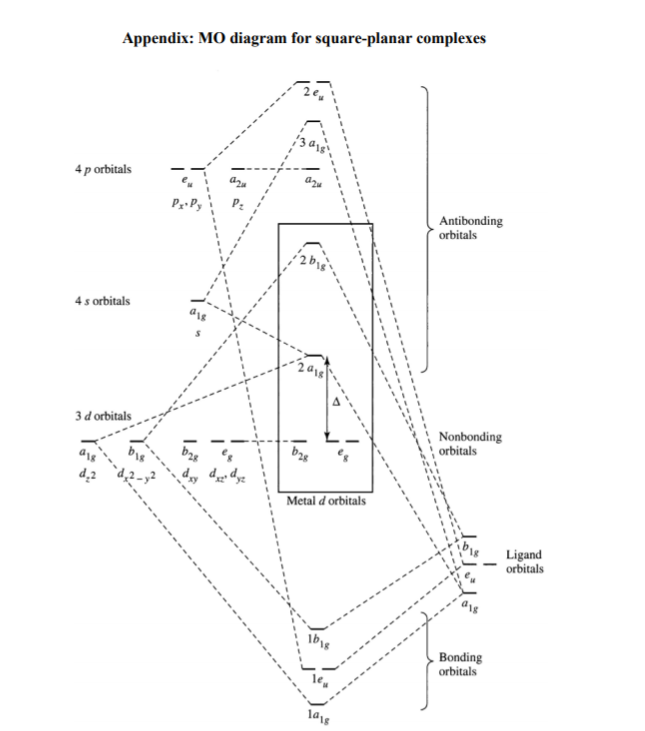
[Solved] Compare the MO diagram of Pt(CN)4 2- and Pt ... Compare the MO diagram of Pt(CN)4 2- and Pt(pyridine)4 2+. Assume the pyridine molecules are all flat in the xy plane. Pyridine is a pi acceptor, but it doesn't have as many pi orbitals as CN- does.
PDF Bonding in transition metal complexes Example: Constructing a MO diagram for Chromium Hexacarbonyl, Cr(CO) 6 Cr ππππ-bonding AOs T2g:(3dxy,3dxz,3dyz) T1u:(4px,4py,4pz) • T2g previouslyconsiderednon-bondingin σ-bondingscheme • T1ucombineswith T1u SALCin in σ-bondingscheme • T1g, T2u π-SALCs are non-bonding Cr non-bonding AOs T2g: (3 dxy, 3dxz, 3dyz) Cr σσσσ-bonding ...
7.3: How to Build Molecular Orbitals - Chemistry LibreTexts General Notes on Molecular Orbital Diagrams. The Y-axis of a MO diagram represents the total energy (not potential nor Gibbs Energy) of the orbitals. Individual atomic orbitals (AO) are arranged on the far left and far right of the diagram. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram.
CN- lewis structure, molecular orbital diagram, and, bond order Also, using the Molecular orbital diagram of CN- we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2.














0 Response to "37 Cn- Mo Diagram"
Post a Comment