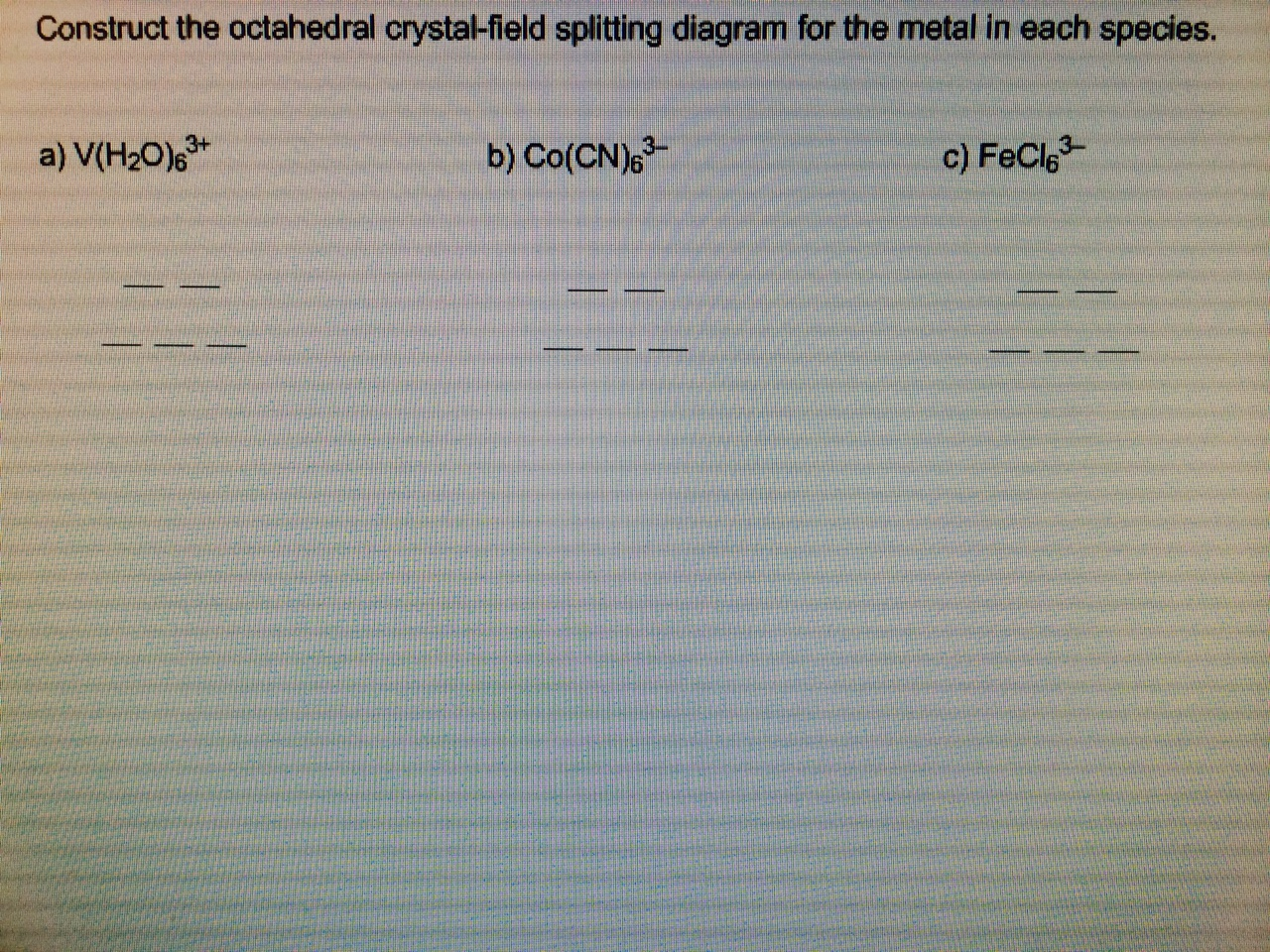
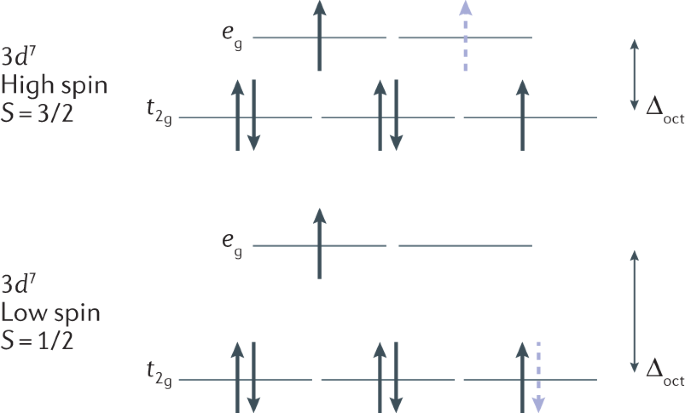
41 construct the octahedral crystal-field splitting diagram for the metal in each species
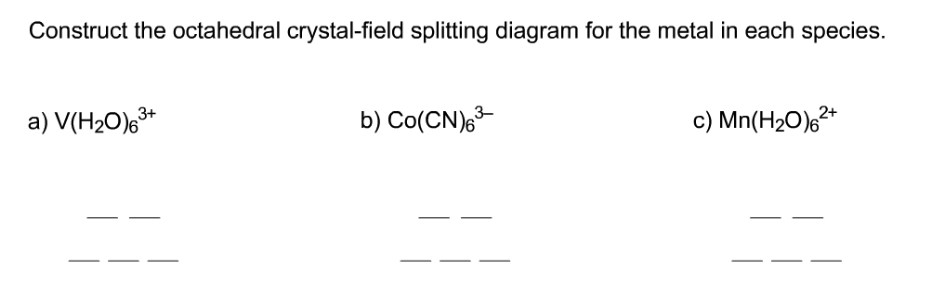
Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
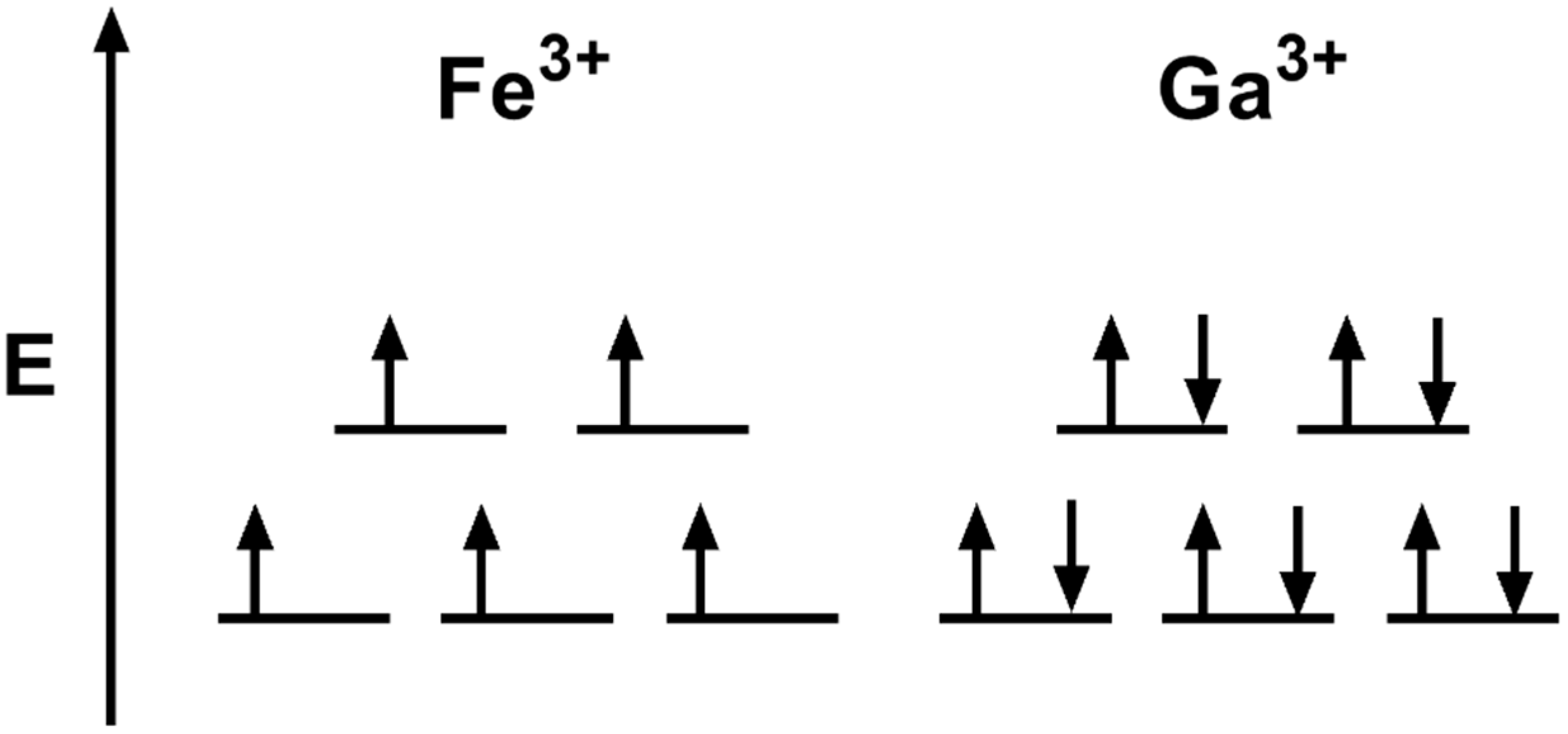
Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+. Nov 14, · Basically, the question is referring to the compound K3 [Fe (C2O4)3]. It asks what is the electron configuration in this comound, I got it to be d5. Fe in the compound is Fe (III) so 23 electrons -> d5.
Construct the octahedral crystal-field splitting diagram for the metal in each species
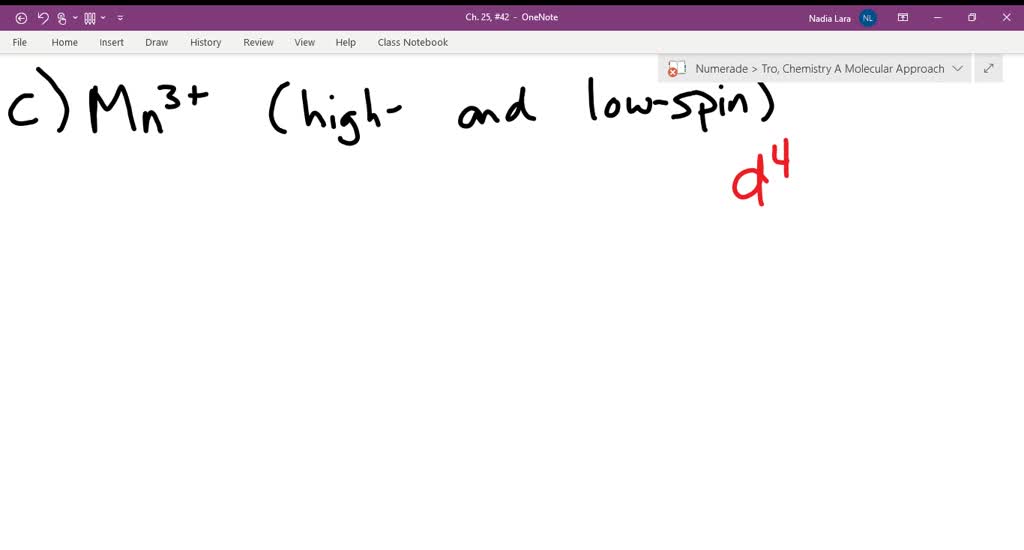
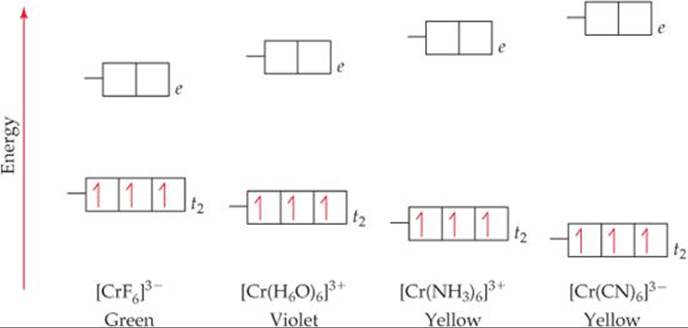
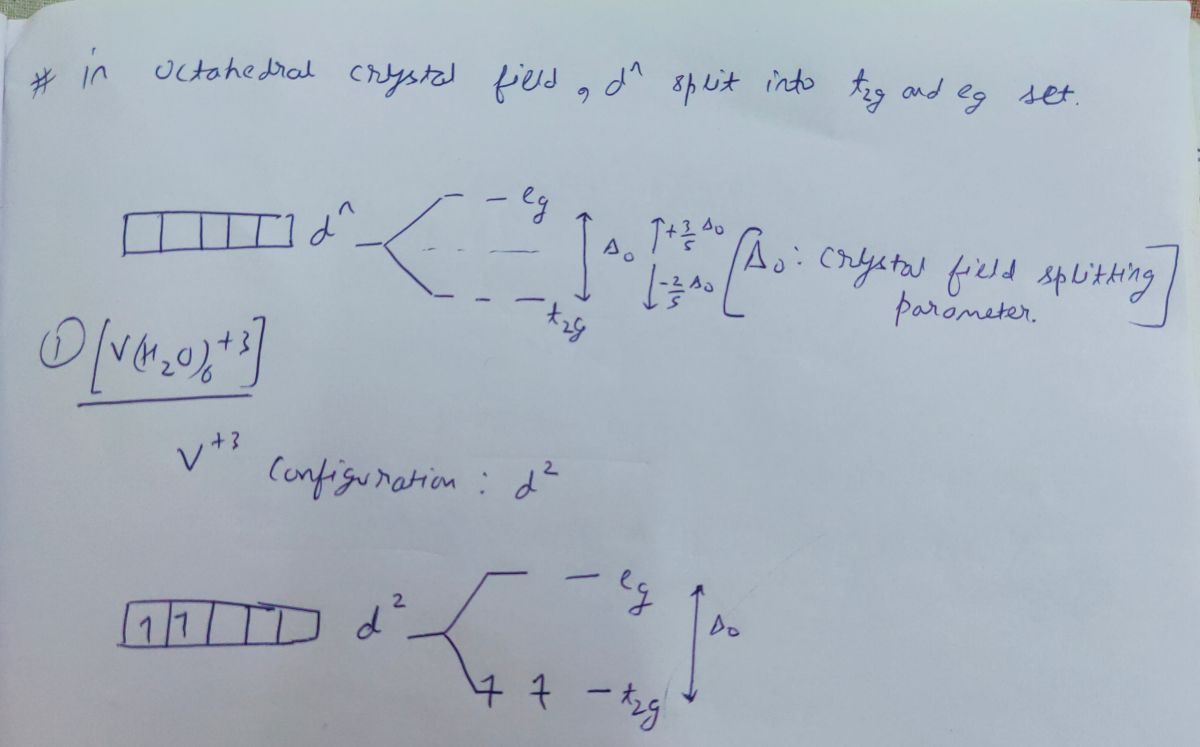
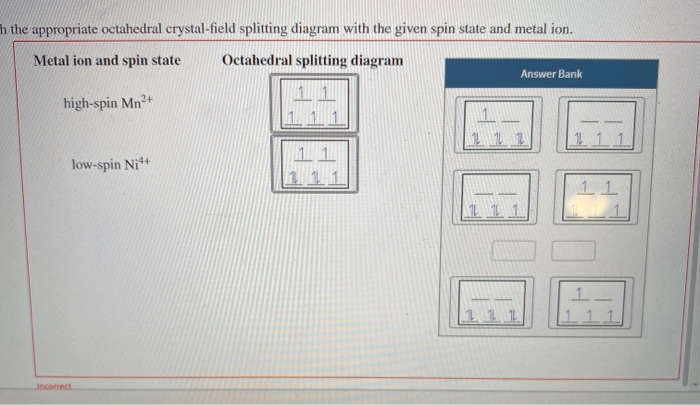
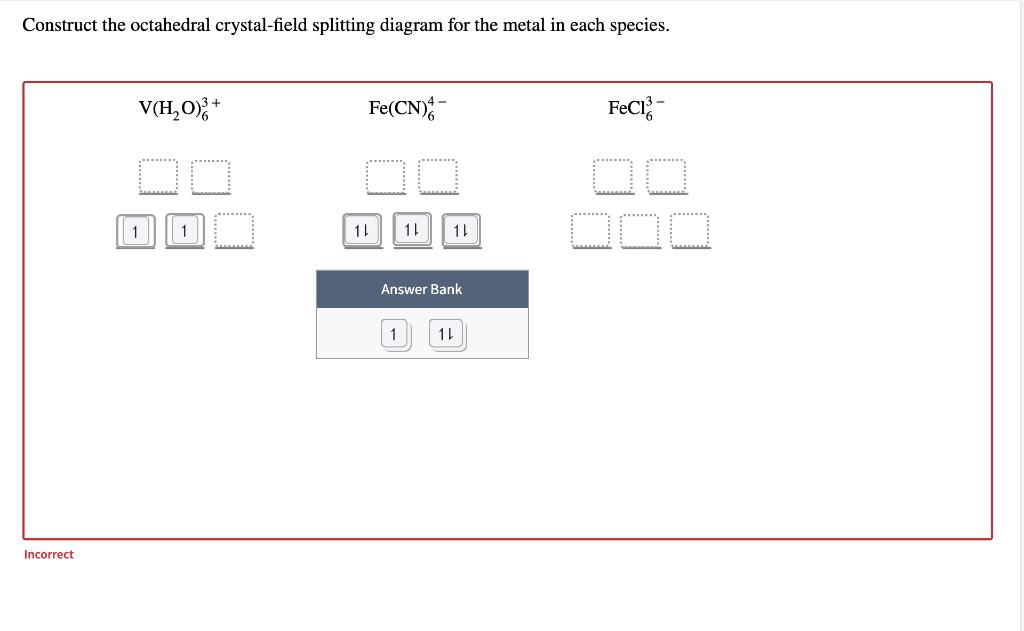
FREE Expert Solution. Octahedral crystal-field splitting diagram → d-orbital electrons. high-spin - electrons can occupy the upper level (eg) low-spin - electrons can pair up with the electrons on the lower level (t2g) Recall that: weak field ligands → high spin → lowΔ or crystal field splitting energy values. This problem has been solved! See the answer. See the answer See the answer done loading. Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V (H2O)63+ Co ( CN ) 3 − 6 Co (CN)63− Mn ( H 2 O ) 2 + 6 Mn (H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in ... Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H_2O)_6^3+ b) Fe(CN)_6^4- c) FeCl_6^3-.
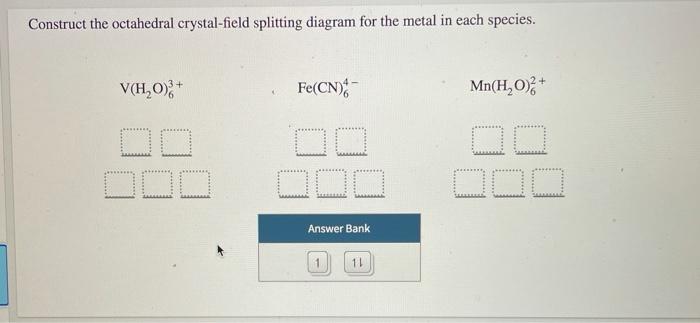
Construct the octahedral crystal-field splitting diagram for the metal in each species. Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. a) V (H20)83* b) Co (CN)8 c) Mn (H2. Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ This board is unable to make drawings/diagrams available. species coefficient is "1" then "1" needs to be entered in ... Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 - Mn(H2O)62+. Construct the octahedral crystal-field splitting diagram for the metal in each species. Question : Construct the octahedral crystal-field splitting diagram for the metal in each species. This problem has been solved! Construct the octahedral crystal-field splitting diagram for the metal in each species. You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop. V(H2O)3+6 Fe(CN)4−6 Mn(H2O)2+6
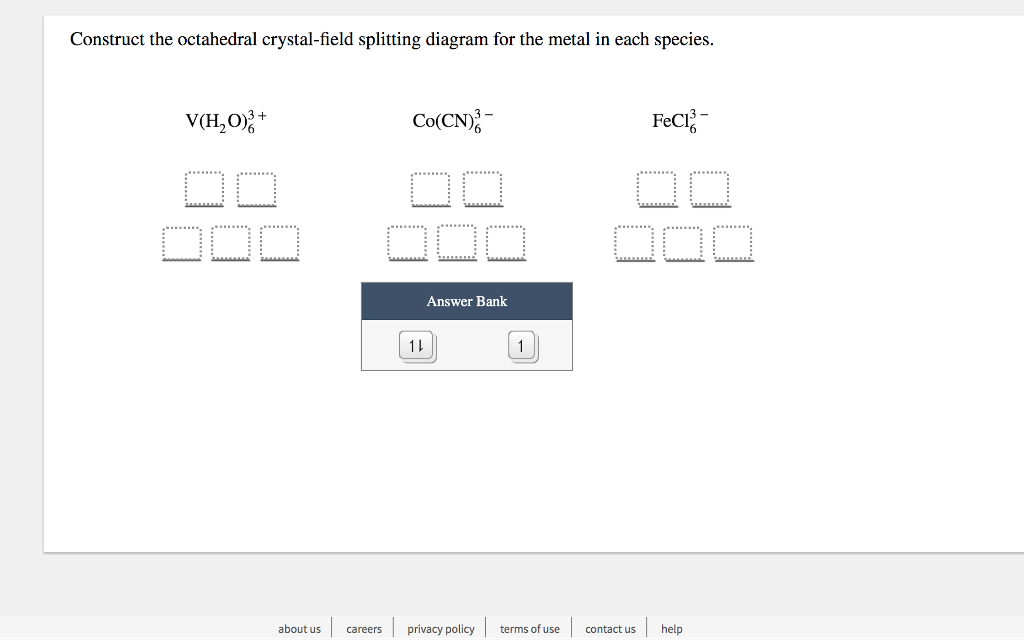
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species, V(H_20)^3+_6 Fe(CN)^4-_6 FeCle^3-_6. Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. This problem has been solved! See the answerSee the ... Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species, a) V(H_2O)_6^3+ Co(CN)_6^3- FeCl_6^3-. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. a) V (H20)83+ b) Fe (CN)6 c) FeCls.
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. a)V(H_2O)6^3+ b)Co(CN)6^3- c) FeCl_6^3-. Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 - Mn(H2O)62+. Transcribed image text: Question 13 of 17 > Construct the octahedral crystal-field splitting diagram for the metal in each species. Construct the octahedral crystal-field splitting diagram for the metal in each species. Balance the equation: Note: If a chemical species coefficient is "1" then "1". Transition elements are typically hard, strong, metals that conduct both heat and electricity very In an octahedral crystal field a low spin d5 Please note: I'm not drawing out ...

construct the octahedral crystal field splitting diagram for the metal in each species vho fecng mnlo amwtr bant 83368
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H_2O)_6^3+ b) Fe(CN)_6^4- c) FeCl_6^3-.
This problem has been solved! See the answer. See the answer See the answer done loading. Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V (H2O)63+ Co ( CN ) 3 − 6 Co (CN)63− Mn ( H 2 O ) 2 + 6 Mn (H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in ...
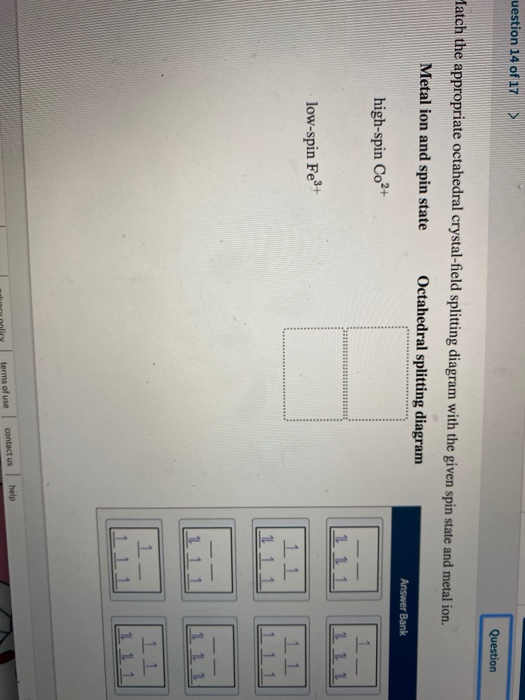
FREE Expert Solution. Octahedral crystal-field splitting diagram → d-orbital electrons. high-spin - electrons can occupy the upper level (eg) low-spin - electrons can pair up with the electrons on the lower level (t2g) Recall that: weak field ligands → high spin → lowΔ or crystal field splitting energy values.
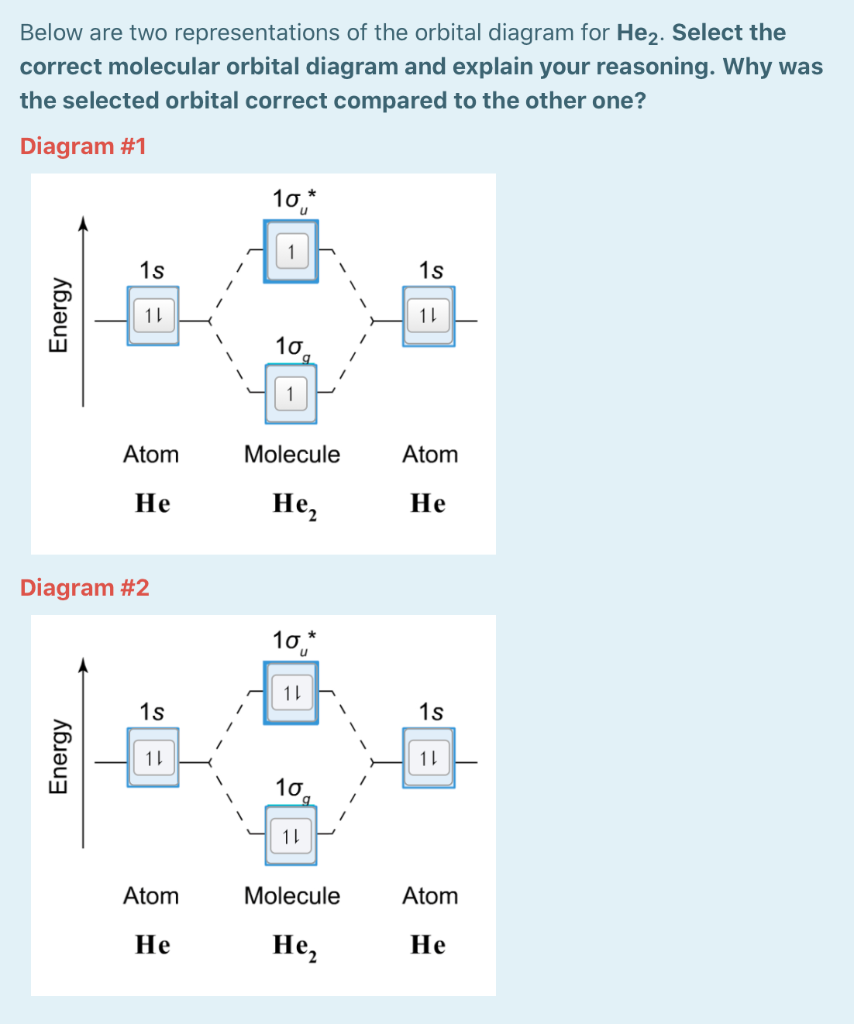







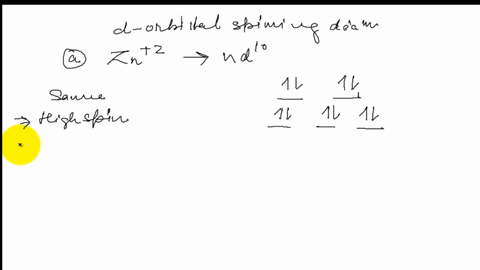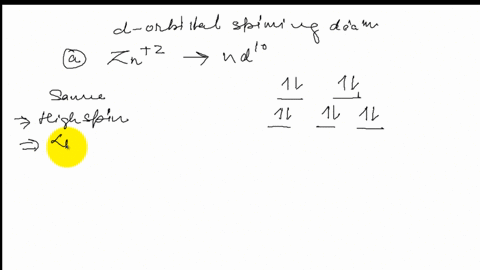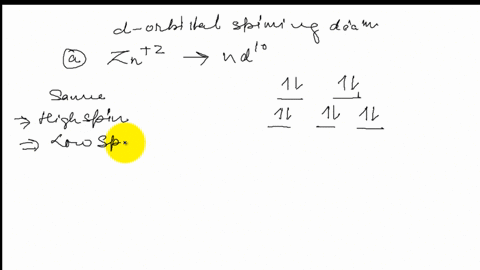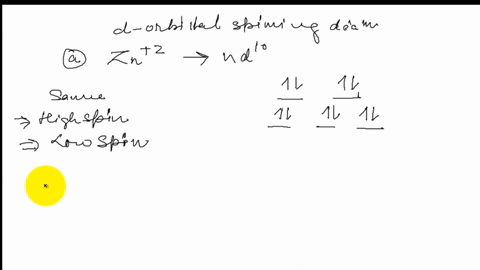











0 Response to "41 construct the octahedral crystal-field splitting diagram for the metal in each species"
Post a Comment