40 orbital diagram of ti
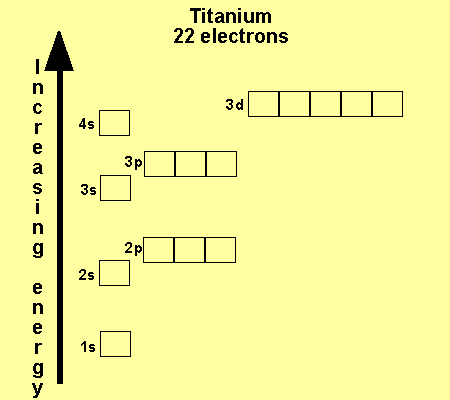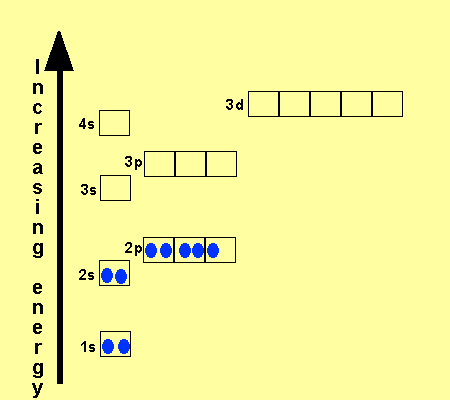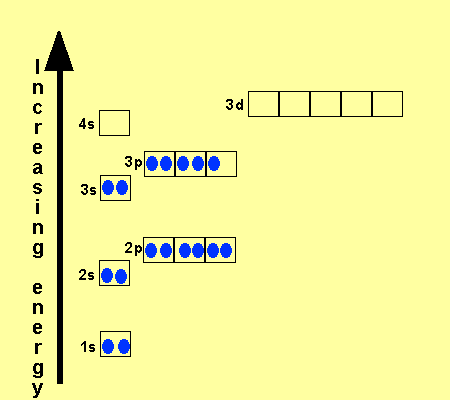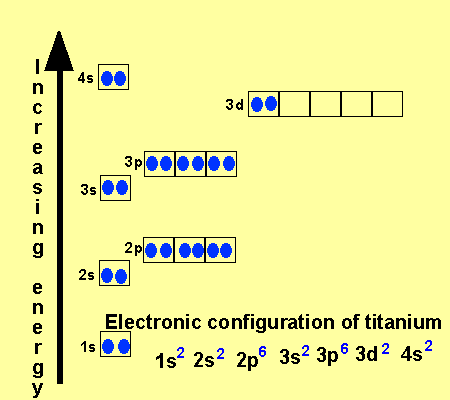
I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi... However, once the 4s orbital is filled, it becomes higher in energy than the 3d orbitals. This means that when titanium loses electrons, it does so from the 4s orbital first. Ti: 1s22s22p63s23p63d24s2
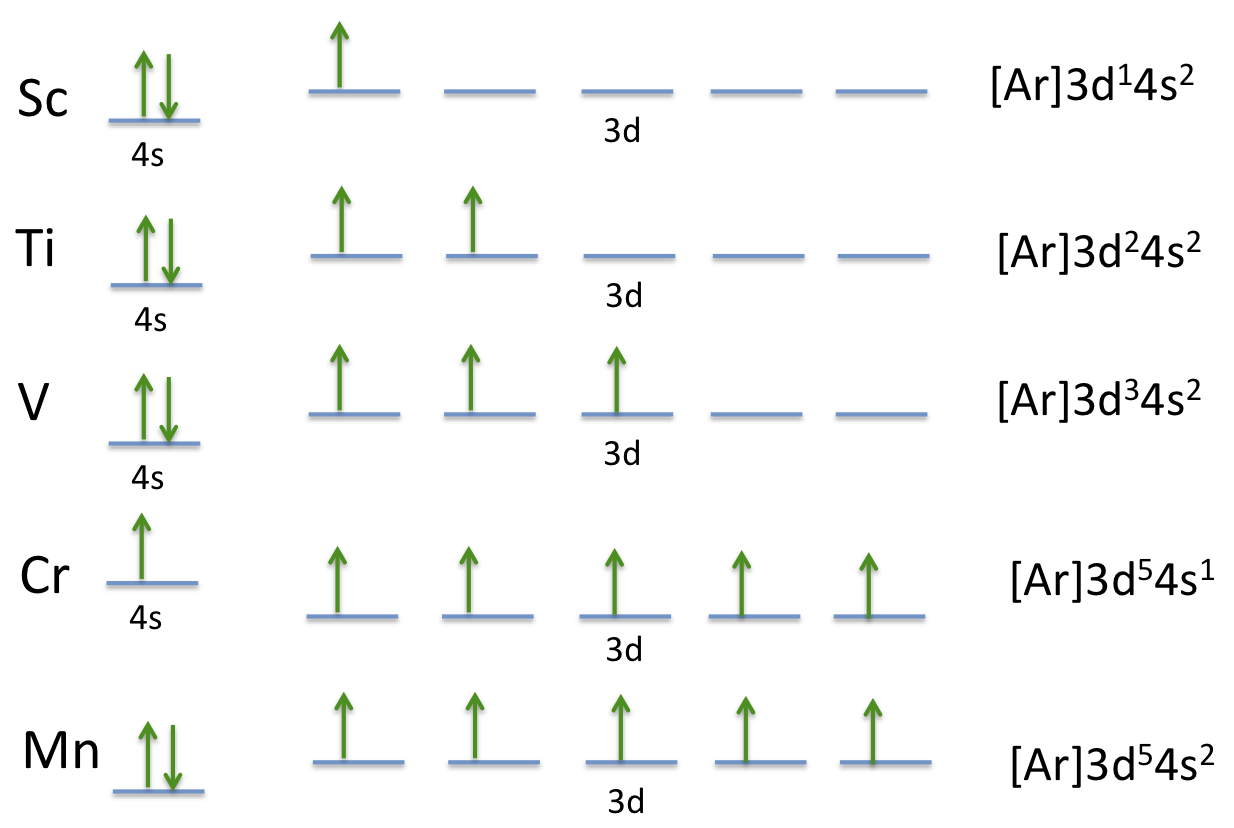
Atomic Orbital Diagram for Chromium (Cr) Chromium ion(Cr 2+, Cr 3+) electron configuration. Ground state electron configuration of chromium(Cr) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1.The electron configuration shows that the last shell of chromium has an electron and the d-orbital has a total of five electrons.

Orbital diagram of ti
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular… Orbital diagram. Zirconium electron configuration ← Electronic configurations of elements . Zr (Zirconium) is an element with position number 40 in the periodic table. Located in the V period. Melting point: 1852 ℃. Density: 6.51 g/cm 3. Electronic configuration of the Zirconium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 2 … The overall molecular orbital energy level diagram for this type of π-bonding in octahedral complexes can be shown as: Figure 21. The generation of π and σ-molecular orbitals in octahedral complexes. Buy the complete book with TOC navigation, high resolution images and
Orbital diagram of ti. I’m a little confused on the connection between a molecules molecular orbital diagram and it’s individual atomic hybridization. Can anyone help me? Thank you Good morning, I'm looking for a way to compute molecular orbital diagrams using fragments or different molecules. I mean something like the diagram linked [here](https://commons.wikimedia.org/wiki/File:H2O-MO-Diagram.svg), where, instead of considering the H2 fragment and the oxygen atom I could put two fragments chosen by me or two molecules. I searched but, at the moment, I didn't find anything useful. I can use Gaussian or Orca for the calculations so if it was possible to obtain such dia... draw a molecular orbital diagram for benzene. Key Terms. Make certain that you can define, and use in context, the key term below. degenerate ; Study Notes. You may wish to review Sections 1.5 and 14.1 before you begin to study this section. Note that the figure showing the molecular orbitals of benzene has two bonding (π 2 and π 3) and two anti-bonding (π * and π 5 *) … Sorry if it's a dumb question, I'm having trouble understanding
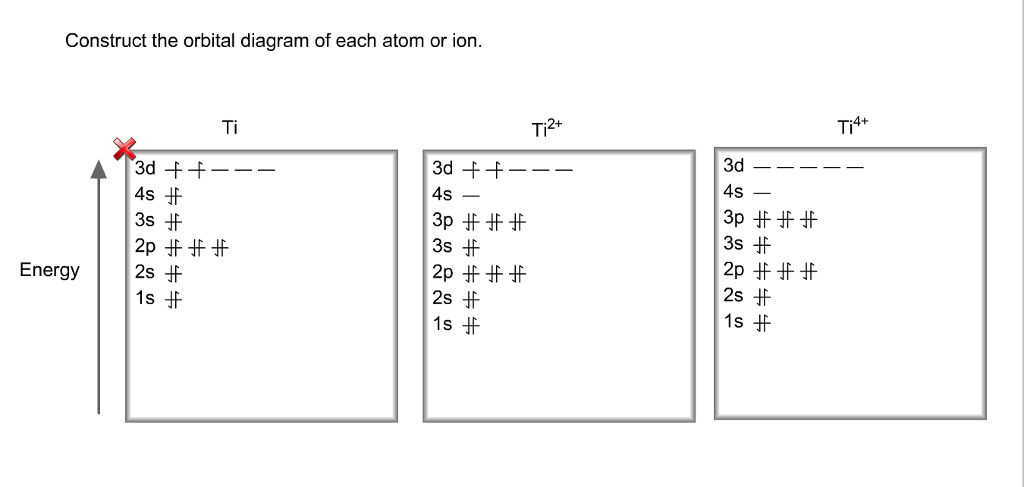
What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts. Nov 20, 2021 · Molecular orbitals diagrams of [Ti (H2O)6]3+. 1. M. O. diagram for [Ti (H2O)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Ti3+→ [Ar] 3d1, 4s0 1e- Ligand group (H2O) orbitals 6 x 2 = 12 e- σ [Ti (H2O)6]3+ molecular orbitals M. O. diagram for [Ti (H2O)6]3+ complex (no π interactions) 01.01.2022 · Density of Titanium (Ti) 4.507 g/cm 3: 23: Density of Vanadium (V) 6.11 g/cm 3: 24: Density of Chromium (Cr) 7.19 g/cm 3: 25: Density of Manganese (Mn) 7.47 g/cm 3: 26: Density of Iron (Fe) 7.875 g/cm 3: 27: Density of Cobalt (Co) 8.9 g/cm 3: 28: Density of Nickel (Ni) 8.908 g/cm 3: 29: Density of Copper (Cu) 8.96 g/cm 3: 30: Density of Zinc (Zn) 7.14 g/cm 3: 31: … What is the orbital diagram of TI? Electrons & Oxidation. Oxidation States. +4,3,2. Electrons Per Shell. 2 8 10 2. Electron Configuration. [Ar] 3d2 4s2. 1s2 2s2 2p6 3s2 3p6 3d2 4s2.
26 Jan 2021 — Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble... Titanium is a transition element which has the atomic number 22. It forms various types of compounds like titanium tetrachloride and titanium... Jun 12, 2018 · Draw the orbital diagram for the d orbitals in an octahedral complex containing. Time-saving video by Brightstorm on Electron Configurations for Transition Metals and Their Ions Problem.The base expression (ground state; Ti0) is [Ar]3d24s2 so removal of 2 electrons to get the oxidation state of 2+ would result in the configuration of [Ar] 3d2.
The second notation groups all orbitals with the same value of n together, corresponding to the "spectroscopic" order of orbital energies that is the reverse of the order in which electrons are removed from a given atom to form positive ions; 3d is filled before 4s in the sequence Ti 4+, Ti 3+, Ti 2+, Ti +, Ti.
What happens to the molecular orbital diagram when a metal-ligand complex is oxidized? Oxidation removes an electron, e.g. you go from d8 metal to d7 metal. As consequence the antibonding orbital has an unpaired electron making the complex less stable (weaker M-L bond, since less pi-backdonation), but how does it change the gap between the metal MO and LUMO of ligand, as well as the gap between the metal MO and HOMO of the ligand?
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Ti (Titanium) is an element with position number 22 in the periodic table. Located in the IV period. Melting point: 1660 ℃. Density: 4.51 g/cm 3 . Electronic configuration of the Titanium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. Electronic configuration of the Titanium atom in ascending order of the levels:
with better orbital overlap. Localization of electrons – narrow bands. Electron hopping between atoms during conduction 1/a a ψ ψ conductivit y E U Na NaNaNa Na +Na-14 U ~I -A ionization energy (a few eV) electron affinity Coulomb repulsion is described in terms of a correlation energy, Hubbard-U,which is the energy penalty for transferring an electron between two adjacent sites. …
For group, there should be a fully filled s sublevel and two electron in the outermost p sublevel. Based on how covalent bonds form with singly filled orbitals, in group14, there are only 2 singly filled orbitals respectively, how can they form the expected 4 bonds? Or does the electron from s move to p to give 4 singly filled orbitals? If that is the case, why does this happen for no reason?
Ti:Sapphire Lasers — 2/11 Each Titanium atom bonds to six neighboring Oxygen atoms, so the Titanium atoms themselves exist effectively as Ti3+ ions. The electronic structure is thus an inert argon shell with a single 3d-orbital electron. In total there are five 3d-orbital
Hey Guys, ​ I was wondering if there are some databases for molexular orbital diagrams of more unusual compounds like phosphaalkenes or sulfur nitrides. I wanted to include some in a presentation ​ thanks for any help!
Exam 4 Review: Ch.8-9. Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye. The energy of one photon of this light is ________ J. Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.19 × 10^14 Hz. Nice work!
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
Bo and Md are electronic parameters calculated by the DV-Xα molecular orbital method proposed by Morinaga et al. [106] to predict the phase stability of Ti alloys. Bo is a bond order that is a measure of covalent bond strength between Ti and an alloying element, whereas Md is a d electron orbital that is related with electronegativity and metallic radius of an alloying element. …
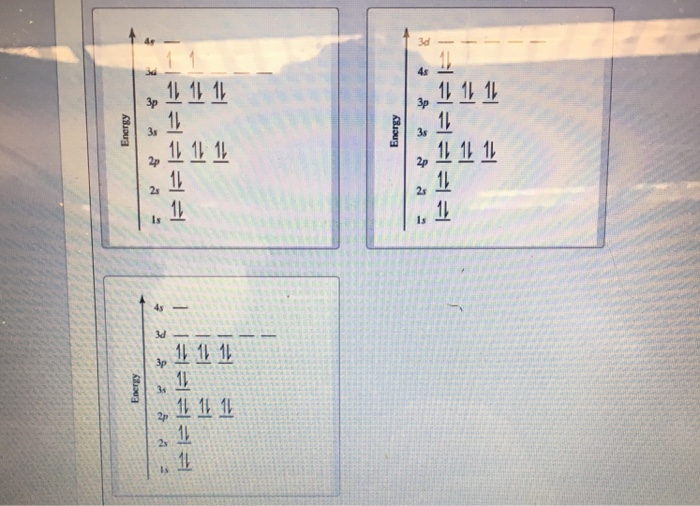
Table 8.3 Partial Orbital Diagrams and Electron Configurations * for the Elements in Period 4. * Colored type indicates the sublevel to which the last electron is added. 8-26. Figure 8.10 A periodic table of partial ground- state electron configurations. 8-27. Figure 8.11.
The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells.
Which principle or rule is violated by the following orbital diagram of an atom in its ground state? ... Sc, Ti, V, Cr. Rank the following atoms in order of the largest to smallest atomic radius: Al, P, Cl, K. K>Al>P>Cl. Below are data on the first four ionization energies for the fictitious element X
Titanium Atomic and Orbital Properties ... Titanium atoms have 22 electrons and the electronic shell structure is [2, 8, 10, 2] with Atomic Term Symbol (Quantum ...
Element reactions. Titanium atoms have 22 electrons and the shell structure is 2.8.10.2. The ground state electron configuration of ground state gaseous neutral titanium is [ Ar ]. 3d2. 4s2 and the term symbol is 3F2. Schematic electronic configuration of titanium. The Kossel shell structure of titanium.
It adds its next electron to the third shell, not the outermost fourth shell. With a configuration of 2-8-10-2, titanium is out in the world and ready to bond with other elements. It makes many natural compounds with halogens and oxygen. Since titanium is out there with four extra electrons, it is quite flexible and forms many compounds.
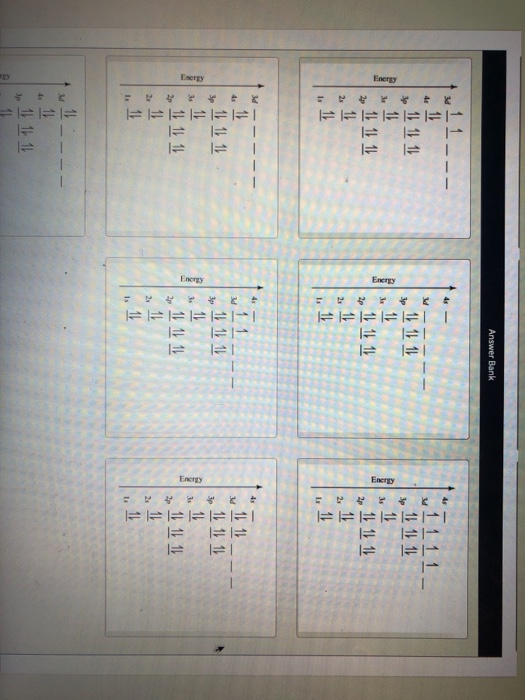
Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is known as.
Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27:
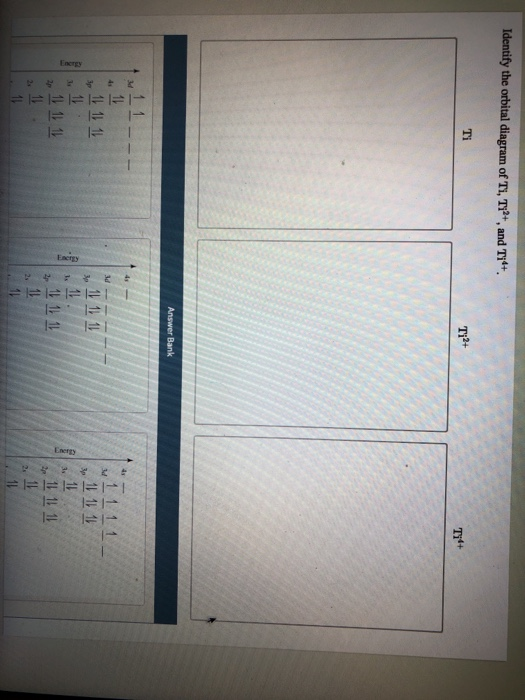
Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+. >
To write the orbital diagram for the Titanium (Ti) first we need to write the electron configuration for just Ti. To do that we need to find the number of e...
I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c...
Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+.
Orbital Diagram. 1s ... 63 Ti: 63: 62.99442(107)# Mass Number The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of nucleons in an atom. Relative Atomic Mass The ratio of the average mass per atom of an isotope to 1/12 the mass of a carbon-12 atom.
4, K, Ca, Sc, Ti ... The electron configuration of an element is a list of the atomic orbitals which ... Each orbital can hold no more than two electrons.
Construct the orbital diagram of each atom or ion. Ti. Ti 2+ Ti 4+ Next. Practice Problems. Choose the orbital diagram that represents the gro Consider the portion of the orbital filling diagra Choose the orbital diagram that represents the gro Identify the element which has the following parti.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Molecular orbital energy-level diagram of anatase TiO2 (adapted from Asahi et al.181). (b) Calculated total density of states (top) and projected density of states for Ti 3d and O 2p orbitals ...
Problem Details. Construct the orbital diagram of each atom or ion. Ti. Ti 2+. Ti 4+. Learn this topic by watching The Electron Configuration: Ions Concept Videos.
Ununennium, also known as eka-francium or element 119, is the hypothetical chemical element with symbol Uue and atomic number 119. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to …
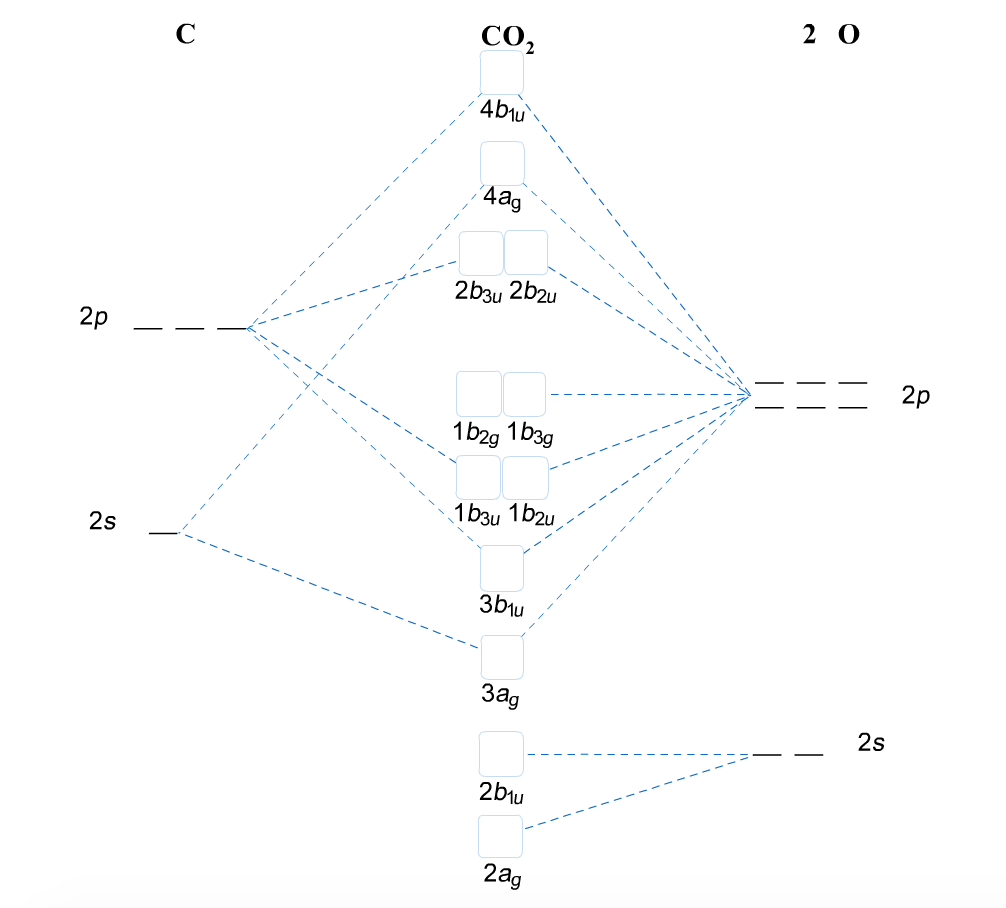
The following diagram shows the positions of these p-orbitals: Since they are out of the plane of the atoms, these orbitals can interact with each other freely, and become delocalized. This means that, instead of being tied to one atom of carbon, each electron is shared by all six in the ring. Thus, there are not enough electrons to form double bonds on all the carbon atoms, but the …
The overall molecular orbital energy level diagram for this type of π-bonding in octahedral complexes can be shown as: Figure 21. The generation of π and σ-molecular orbitals in octahedral complexes. Buy the complete book with TOC navigation, high resolution images and
Orbital diagram. Zirconium electron configuration ← Electronic configurations of elements . Zr (Zirconium) is an element with position number 40 in the periodic table. Located in the V period. Melting point: 1852 ℃. Density: 6.51 g/cm 3. Electronic configuration of the Zirconium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 2 …
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…





/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)

















0 Response to "40 orbital diagram of ti"
Post a Comment