40 iodine electron dot diagram
Hint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, if they exist in the molecule. Iodine is the molecule consisting of two atoms of iodine bonded together satisfying both the planes. Complete answer: Let us study the concept; A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Iodine Lewis Dot Diagram. Here are a number of highest rated Iodine Lewis Dot Diagram pictures upon internet. We identified it from obedient source. Its submitted by dispensation in the best field. We agree to this kind of Iodine Lewis Dot Diagram graphic could possibly be the most trending subject gone we share it in google lead or facebook.

Iodine electron dot diagram
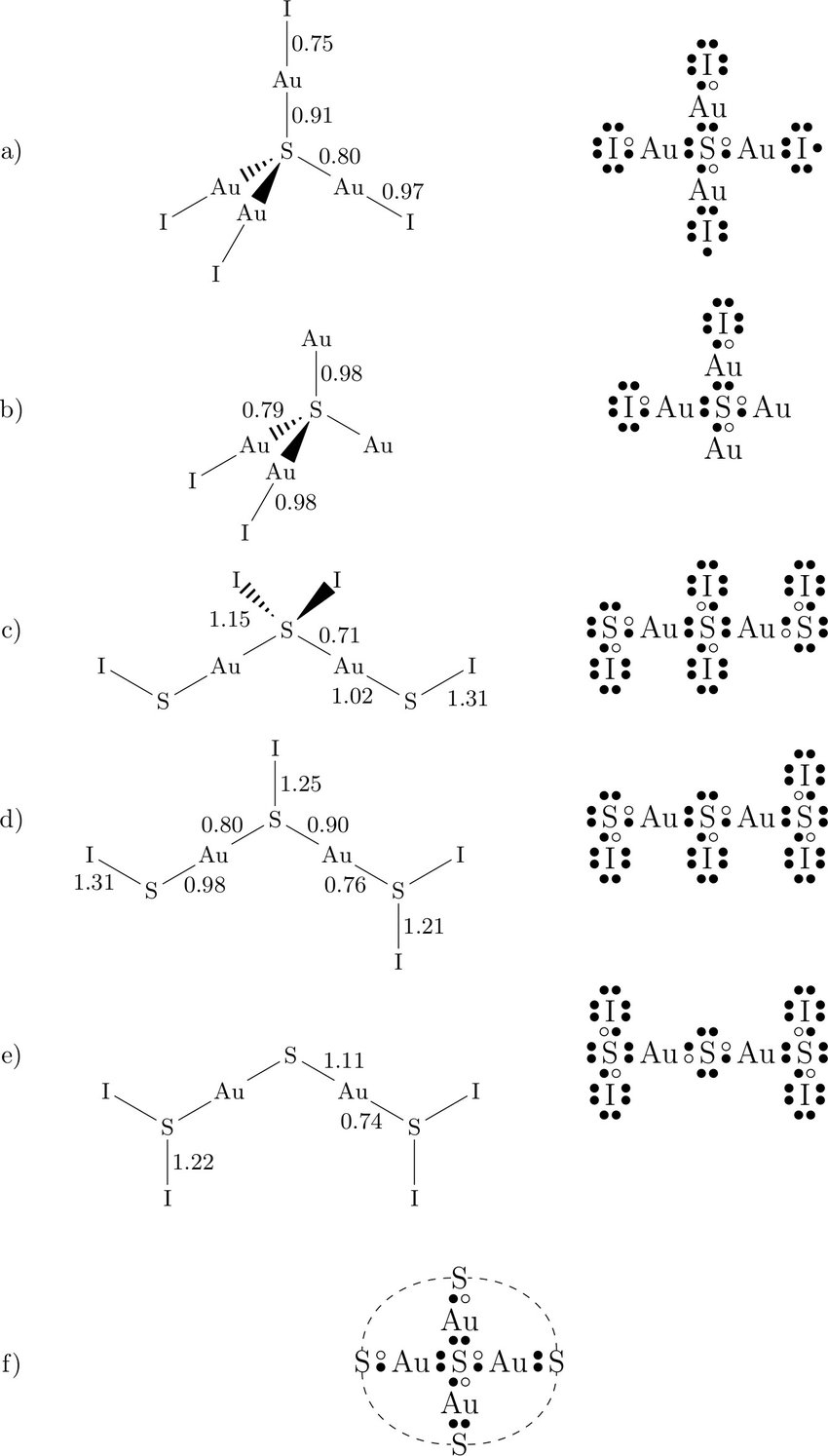
Hence, the Lewis structure of iodine trichloride would be: We can observe that every chlorine atom is surrounded by eight electrons but a central atom, iodine, is surrounded by ten electrons. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons ... Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model .
Iodine electron dot diagram. Precautions for safe handling: Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection. Sigma-Aldrich; Safety Data Sheet for Iodine Monobromide. Product Number: 224847, Version 4.5 (Revision Date 08/22/2014). Cyanogen iodide may cause convulsions, paralysis and death from respiratory failure. It is a strong irritant and may cause burns to the eyes and skin if contacted. If cyanogen iodide is heated enough to undergo complete decomposition, it may releases toxic fumes of nitrogen oxides, cyanide and iodide. A fire may cause the release of poisonous gas. An electron dot diagram can show you that the symbols for an element surrounded by dots. Each dot stands for one valence electron. How many dots are shown in the electron dot diagram of iodine? Lewis structure of iodine molecule contains only one I-I bond and each iodine atom has three lone pairs. It is very easy to draw the I 2 lewis structure. I 2 lewis structure. There is only a single bond between iodine atoms and three lone pairs on each iodine atoms. So, this lewis structure is a very simple.
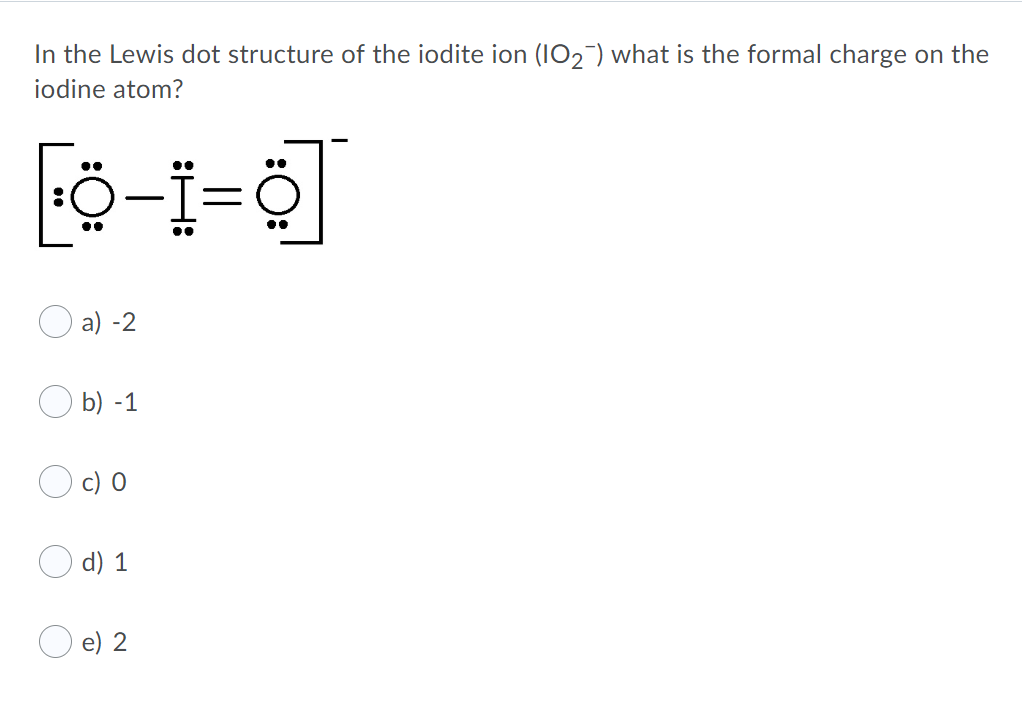
For the Iodine--Iodine has 7 valence electrons on the periodic table. Nonbonding, we have 2 right here that are not involved in a chemical bond. Minus bonding, we have 2, 4, 6, which we'll divide by 2. That gives us a +2 formal charge for the Iodine. For the Oxygens, they're all the same so we'll just do one. For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. We have a total of 14 valence electrons. We'll put 2 between atoms to form the chemical bond, and we'll go around the outside. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... Draw the electron dot structure … 23)Draw a Lewis electron-dot diagram for a molecule of phosphorus trichloride, PCl3 Thus in comparing the electron ...
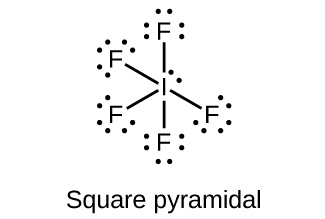
Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you' ...Oct 25, 2016 · Uploaded by Wayne Breslyn ICl2- lewis structure contains one iodine atom at the middle position whereas two chlorine atoms at the surrounding position. There are three lone pairs present on the central atom of ICl2- lewis structure. Also, the iodine central atom in ICl2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell. Lewis Structure Lewis structure is the representation of the electrons of the molecules. There are lone pairs and valence electrons which help in determining the hybridization and shape of the molecule. As there are molecules of Iodine, one molecule of Iodine will be in the centre. Also, iodine is in the seventh group of the periodic table and ... 1 answerWhat should the electron dot diagram for iodine look like? Chemistry Drawing Lewis Structures. 1 Answer. Jahan Psyche. Oct 24, 2015. peoi.org.
To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s 2 2s 2 2p 6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
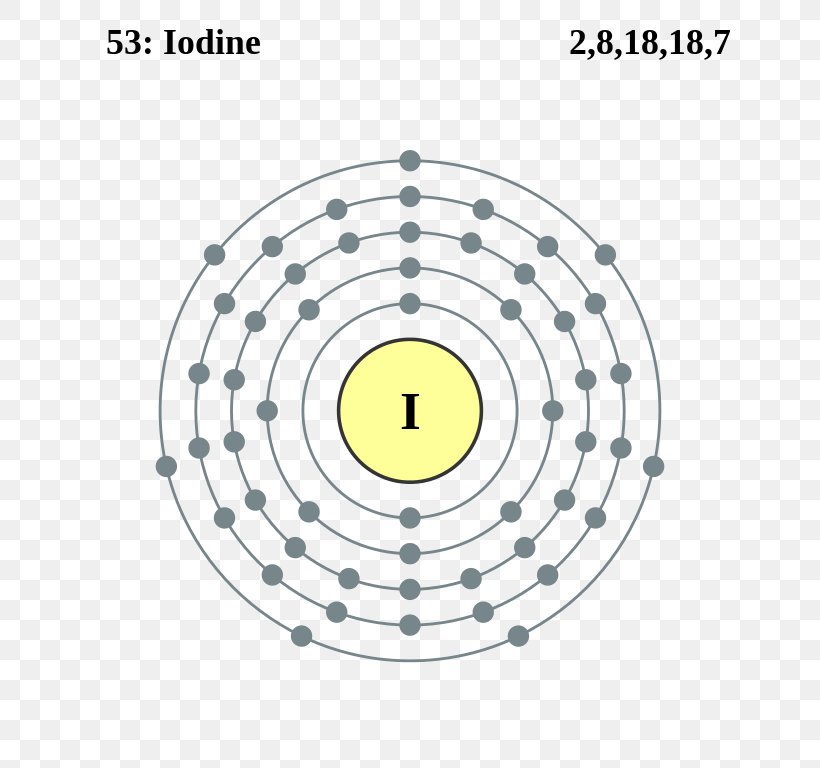
Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. ... Atomic Structure of Iodine. ... Electron Dot Model. Chemical Properties of Iodine. Electrochemical Equivalent: 4.7348g/amp-hr;
iodine has 7 valence electrons. So you draw seven dots, 2 on each side of the letter I. and on one side you just put one dot, I think its best to put. The left diagram shows a Lewis dot structure of sodium with. ASK A BRAND Likewise, they can be used .. The left shows an iodine atom with one lone pair.
Iodate ion contains one iodine and three oxygen atoms. Lewis structure of iodate ion (IO 3-) contains two I=O bonds and one I-O bond. There is -1 charge on oxygen atom in IO 3-lewis structure. IO 3-lewis structure. Oxygen atoms have made bonds with center iodine atom. From those bonds, there are two double bonds and one single bond in the IO 3 ...
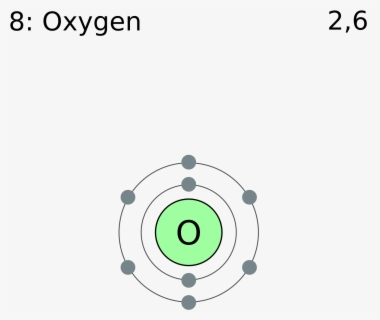
The electronic configuration of iodine is [Kr]4d105s25p5. As we can see, there are seven electrons in the valence shell of iodine. Secondly, the electronic configuration of chlorine is 1s22s22p63s23p5. Since chlorine and iodine belong to the same group i.e. group 17, there are seven electrons in the valence shell of chlorine as well.
Iodine is a diatomic molecule so its molecules are paired as i2 iodine has 7 electrons in its outer shell so 1 electron is shared by each atom. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for. Chemical properties of iodine.
Apr 28, 2021 — Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the ...
A step-by-step explanation of how to draw the I Lewis Dot Structure.For the I structure use the periodic table to find the total number of valence electrons ...
Hydrogen and Iodine are both non-metals, so they form a COVALENT bond and SHARE electrons to complete their outer shells. Hydrogen shares one electron with i...
NaCl's Lewis structure: ... What is the ionic compound formed from calcium and iodine? Calcium - metal - 2 valence electrons - loses both electrons [Ca]+2. Iodine - nonmetal - 7 valence electrons - gains 1 electron ... Lewis Diagram of Calcium Iodide ...
In a Lewis structure, atoms that are bonded covalently are represented by a single line joining the two atoms, which are represented by the element's chemical symbol. Covalent bonds occur mainly in diatomic molecules, such as hydrogen, nitrogen, fluorine, chlorine, bromine, iodine, and astatine.
Iodine monochloride, 99.998% trace metals basis. Q414607. Iodine monochloride solution, 1.0 M in methylene chloride. Iodine monochloride, 1M solution in dichloromethane, AcroSeal (R) Iodine monochloride, ACS reagent, 1.10+/-0.1 I/Cl ratio basis. Iodine monochloride, approx. 0.22N soln. in glacial acetic acid.
This example Total valence electrons to be "happy" = 1 iodine (8) + 3 chlorine (3 x 8). Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used ..
CHEMISTRY LAB: ELECTRON DOT DIAGRAMS FOR IONIC COMPOUNDS WHAT TO TURN IN: Data Table Questions #1-5 Objectives • To review element and ion names and symbols ... magnesium and iodine MgI 2 3) aluminum and fluorine AlF 3 4) calcium and nitrogen Ca 3N2 5) zinc and selenium ZnSe (Note: zinc is a typical transition metal.) ...
Iodine dot diagram is actually amongst images libraries inside of our highest photos gallery. I wish you are going to revel in. We're going to draw the Lewis structure for I2, Iodine gas, a very pretty purple gas. So let's start out. Iodine is in group 7 of the periodic table. That means it has 7 valence electrons, so we have 7.
The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model .
Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
Hence, the Lewis structure of iodine trichloride would be: We can observe that every chlorine atom is surrounded by eight electrons but a central atom, iodine, is surrounded by ten electrons. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons ...







/Iodine-58b601a35f9b5860464bfeb4.jpg)








0 Response to "40 iodine electron dot diagram"
Post a Comment