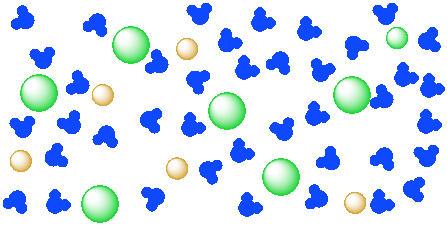
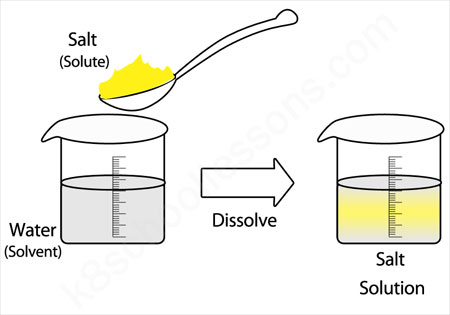
40 diagram of salt dissolved in water
3. Salt dissolves in water. Describe how you could obtain pure salt from a salt solution. Draw a diagram of the apparatus you would use The amount of air that can be dissolved in water increases with pressure and decreases with temperature. Deaeration. When fresh water is heated up air bubbles start to form. The water can obviously not hold the dissolved air with increased temperature. At 100 o C (212 o F) water starts to boil - the bubbles are formed by evaporated water or ...
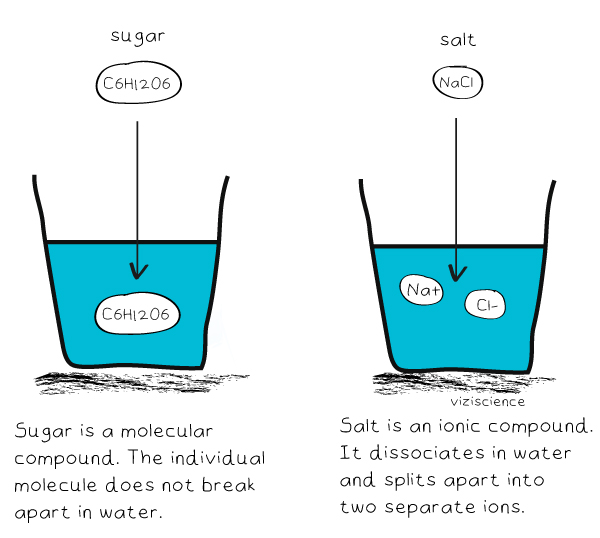
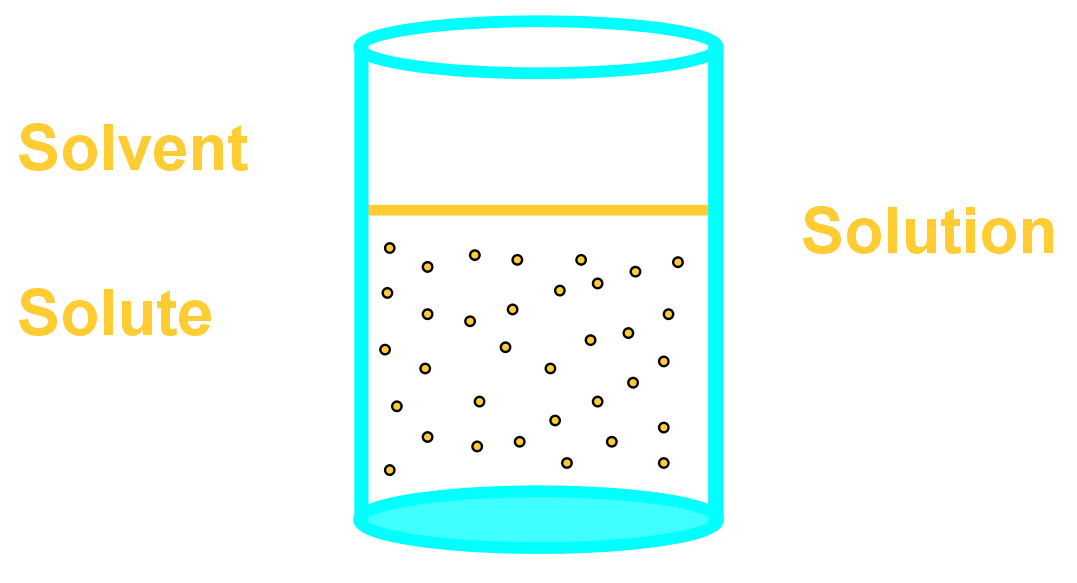
Salt molecules are very charged, so they dissolve easily in water, which has slightly charged molecules. Salt dissolves less easily in alcohol, because alcohol molecules have less charge than water. Alcohol also has a portion of its molecule that has no charges, i.e., it is non-polar, like oil.
Diagram of salt dissolved in water
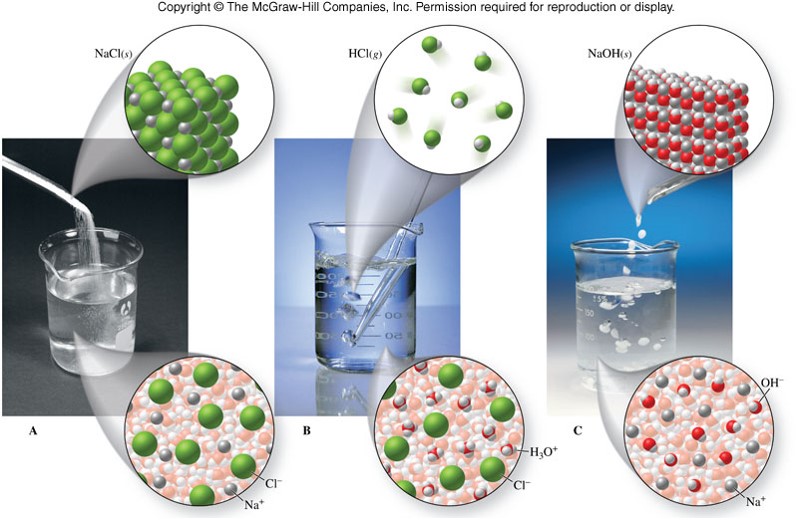
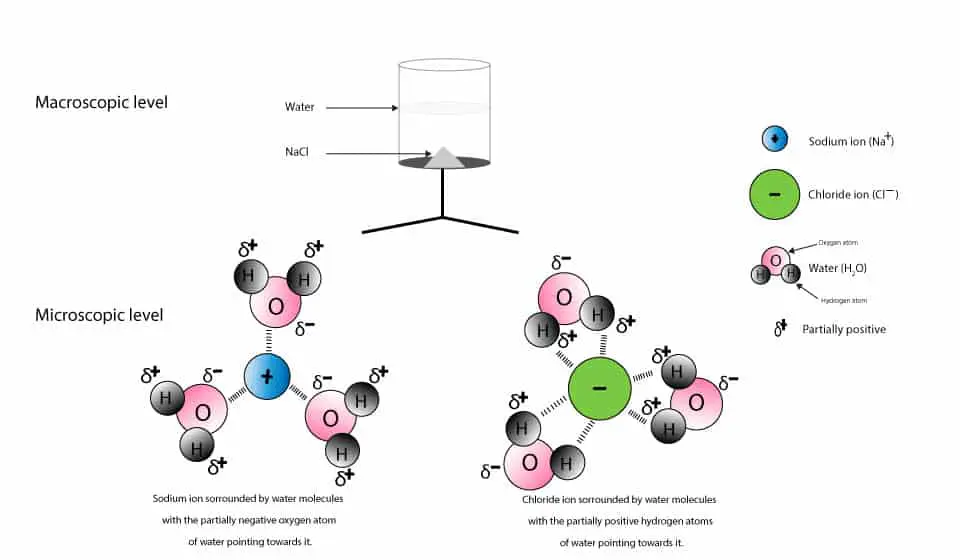
An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. For example, sodium (Na) and chlorine (Cl) form an ionic bond to make NaCl (table salt). However, in a covalent bond, the atoms are bound to share electrons. For example, if we talk about water ( H2O), it is a polar covalent bond. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Does salt disappear when dissolved in water? Salt dissolves in water because water molecules have one negative end and one positive end. (c) The solubility of the salt in a water is defined as : the maximum amount of the salt which is dissolved in 100 g of water (or any other solvent) to form a saturated solution at a given temperature In the light of this, at 293 K (d) With rise in temperature, the solubility of all the salts in water increases.
Diagram of salt dissolved in water. Phase Diagrams and Salt Crystallization. During solar evaporation, salt crystal nucleation and growth take place on the evaporating surface as the concentration of solution increases. 36 Meanwhile, the salt ions diffuse back to bulk liquid from the heating surface due to the concentration gradient. 37 Inadequate dissipation of the salt results in clogged surfaces that increase solar reflection ... Oct 12, 2019 · The water filtration process begins by pushing water through a pre-filter, also known as a sediment filter. The process removes dissolved solids (TDS) like salts from the water. It removes about 90-99% of the salts, leaving the solid waste behind in the reject stream. Post this; the booster pump works by building water pressure. Looking at it from a molecular perspective, salt dissolves in water as a result of an electrical charge. Moreover, both water and salt compounds are polar, featuring negative and positive charges on the molecular opposite side. Salt has bonding compounds called ionic, which have an electric charge in both negative and positive ends. The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell?
Salinity means the total content of dissolved salts in Sea or Ocean. Salinity is calculated as the amount of salt dissolved in 1,000 gm of seawater. It is generally expressed as 'parts per thousand' (ppt). A salinity of 24.7 % has been regarded as the upper limit to fix 'brackish water'. It is a significant factor in deciding several ... Reverse Osmosis works by using a high pressure pump to increase the pressure on the salt side of the RO and force the water across the semi-permeable RO membrane, leaving almost all (around 95% to 99%) of dissolved salts behind in the reject stream. The amount of pressure required depends on the salt concentration of the feed water. Correct answers: 2 question: Salt is dissolved into a water solvent. Can the salt be extracted, and will it retain its properties? (1 point) O No, it cannot be extracted through any means. It retains its original properties. Yes, it can be extracted through evaporation. It retains its original properties. O No, it cannot be extracted through any means. It does not retain its original ... At a particular temperature, the solubility of O2 in water is 0.500 M when the partial pressure is 0.120 atm. What will the solubility be when the partial pressure of O2 is 0.360 atm? The predicted van't Hoff factor for a calcium chloride solution, CaCl2 (aq), is
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.Once this happens, the salt is dissolved, resulting in a homogeneous solution. When no more salt dissolves in water at a particular temperature, then the solution at that temperature is called (a) unsaturated (b) saturated (c) super saturated (d) none of the above. Answer. Answer: (b) saturated. Question 29. The process of conversion of water into vapour is called (a) evaporation (b) condensation Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Ammonia is a key component of the nitrogen cycle in streams, where it may be dissolved in the water column or associated with sediments. At high enough concentrations, ammonia can be toxic to aquatic organisms. In general, unionized ammonia (NH 3) is the form most toxic to aquatic biota.
Water and salt are both ionic and, when dissolved, their structures get aligned according to polarity. The positive end of water that; the hydrogen is attracted to the Chloride atoms of the salt. Similarly, a negative end of a water molecule is an Oxygen atom; that attracts the Sodium part of salt.
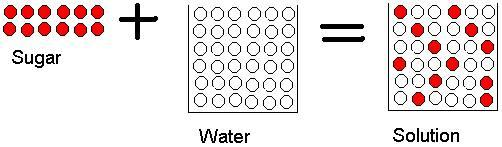
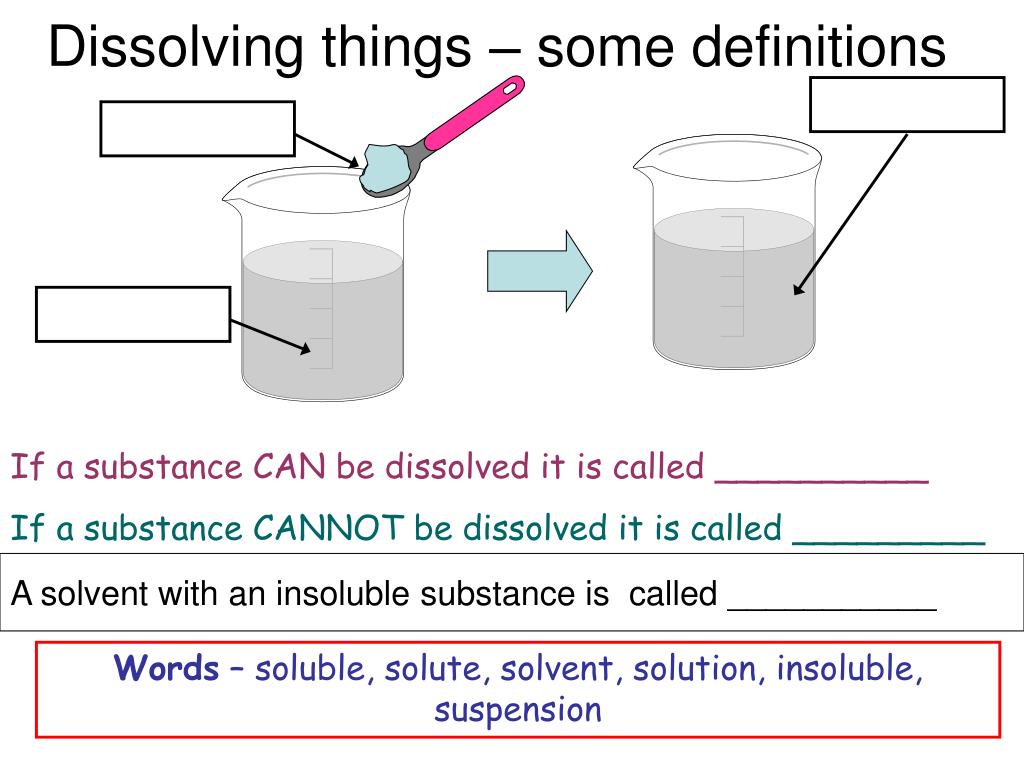
Salt and sand are dissolved in water. Salt is soluble but sand being insoluble, settles and can be separated by sedimentation, decantation followed by evaporation of salt water. Class 6 Science Chapter 5 Separation of Substances Additional Important Questions and Answers. Very Short Answer Type Questions. Question 1.
She stirs a tablespoon of salt into a cup of warm water and watches the salt dissolve. Why does the salt dissolve in water? The partial positive charges on hydrogen atoms in water molecules are attracted to chlorine ions, and the partial negative charges on oxygen atoms in water molecules are attracted to sodium ions.
Salinity is the measurement of the amount of salt dissolved in a water body. We can measure this value by dividing the gram amount of salt in the given sample from kilogram amount of seawater. Salinity is an important parameter in determining many aspects regarding the chemistry of natural water and the biological processes within the water body.
After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Type or paste a DOI name into the text box. Click Go. Your browser will take you to a Web page (URL) associated with that DOI name. Send questions or comments to doi ...
Answers. Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
5. The graph below shows the amount of salt that dissolves in 100 milliliters (mL) of water at various temperatures. Based on the graph, what is the likely amount of salt that will dissolve in 200 mL of water at 90°C? A. 35 grams B. 40 grams C. 80 grams D. 100 grams 6. The graphic shows sugar in a cup before and after it has dissolved.
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
A. The molecules of water and salt are charged, making them reactive. B. Oil molecules do not contain any charge so salt cannot be dissolved. C. Salt dissolves in water because water is reactive while oil is nonreactive. D. Salt and oil are not chemically alike, while water molecules interact with the charged salt molecules.
Example of a detailed conceptual model diagram for dissolved oxygen. Click on the diagram to view a larger version. Aerobic aquatic life requires oxygen for survival, and most are dependent upon oxygen dissolved in the water column. Dissolved oxygen (DO) concentrations are normally sufficient to maintain healthy biotic assemblages in unpolluted ...
Answer: Due to salt particles it is more difficult for water molecules to freeze back onto the ice, ice in contact with dissolved salt melts more quickly. When saltwater travels over the ice ball's surface, it melts the ice along the route, forming channels that seem like rivers. chemical reacti...
Compared to filter sand and some other filtering media, you can retain much more water flow with less pressure loss using Turbidex. The media has surface micro-mineral projections (with 0.25 to 10 µm spacing that effectively traps suspended solids. This, along with the high surface area, make Turbidex an ideal water filtration media.
The diagram shows salt dissolved in water. What does it show about water molecules - 20635482 simonenekia2000 simonenekia2000 01/14/2021 Chemistry College answered The diagram shows salt dissolved in water. What does it show about water molecules and chloride ions? + Chloride Oxygen Hydrogen 4 Sodium 1

Image from page 141 of "The Civil engineer and architect's journal, scientific and railway gazette" (1839)
(c) The solubility of the salt in a water is defined as : the maximum amount of the salt which is dissolved in 100 g of water (or any other solvent) to form a saturated solution at a given temperature In the light of this, at 293 K (d) With rise in temperature, the solubility of all the salts in water increases.
After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Does salt disappear when dissolved in water? Salt dissolves in water because water molecules have one negative end and one positive end.

Image from page 421 of "The open door to independence; making money from the soil; what to do--how to do, on city lots, suburban grounds, country farms, together with outline maps of all parts of the United States, irrigated regions, climates, cities, vil
An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. For example, sodium (Na) and chlorine (Cl) form an ionic bond to make NaCl (table salt). However, in a covalent bond, the atoms are bound to share electrons. For example, if we talk about water ( H2O), it is a polar covalent bond.



.png)















0 Response to "40 diagram of salt dissolved in water"
Post a Comment