37 orbital diagram of silicon
26.01.2021 · How Many Valence Electrons are in Silicon. There are 4 electrons in the outer shell of Silicon so the number of valence electrons in silicon is 4. Silicon Orbital Diagram. Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. … Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Given: Silicon atom. An orbital diagram refers as the arrangement of the electrons in an atom. In the orbital diagram, the each orbital is shown as square and in a sublevel they are represented next to each other in a horizontal way. Silicon [N e] 3 s ² 3 p ²
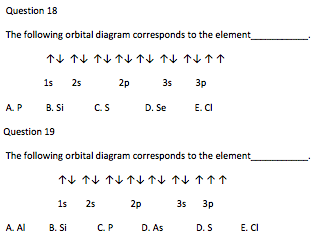
Orbital diagram of silicon
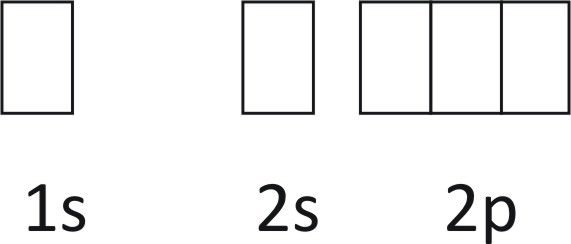
Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2.The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. 01.11.2021 · Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10
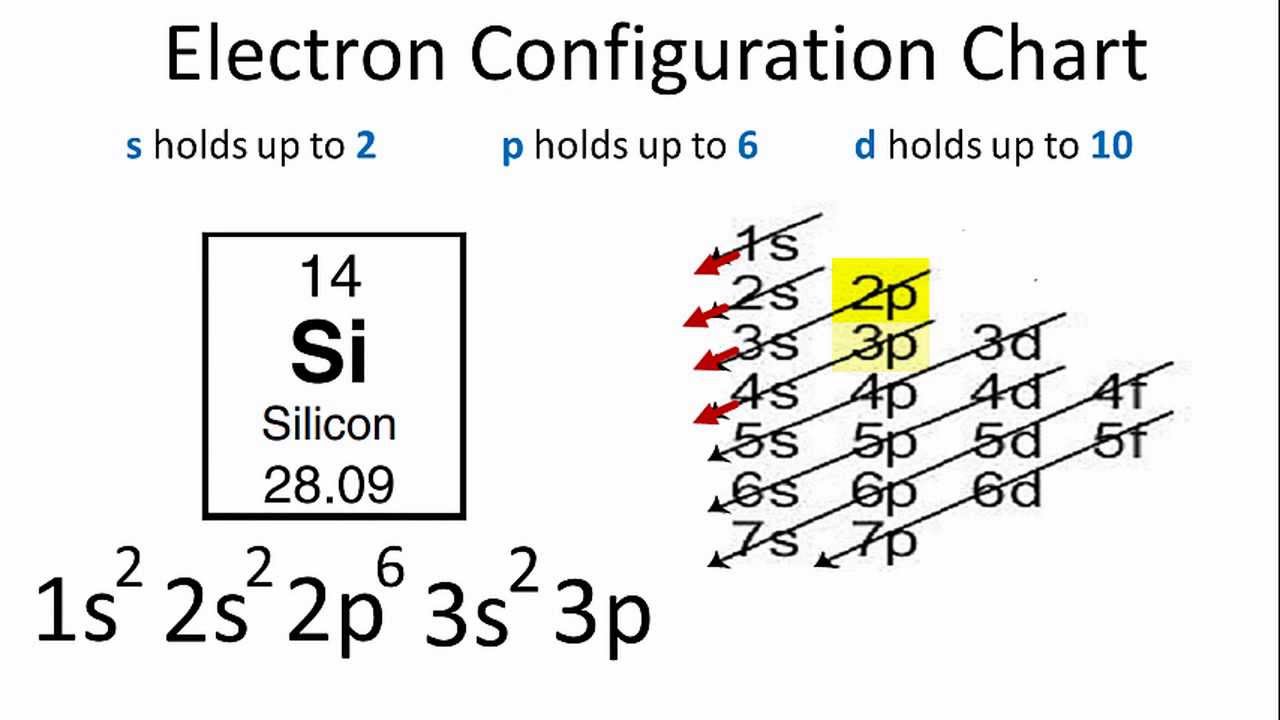
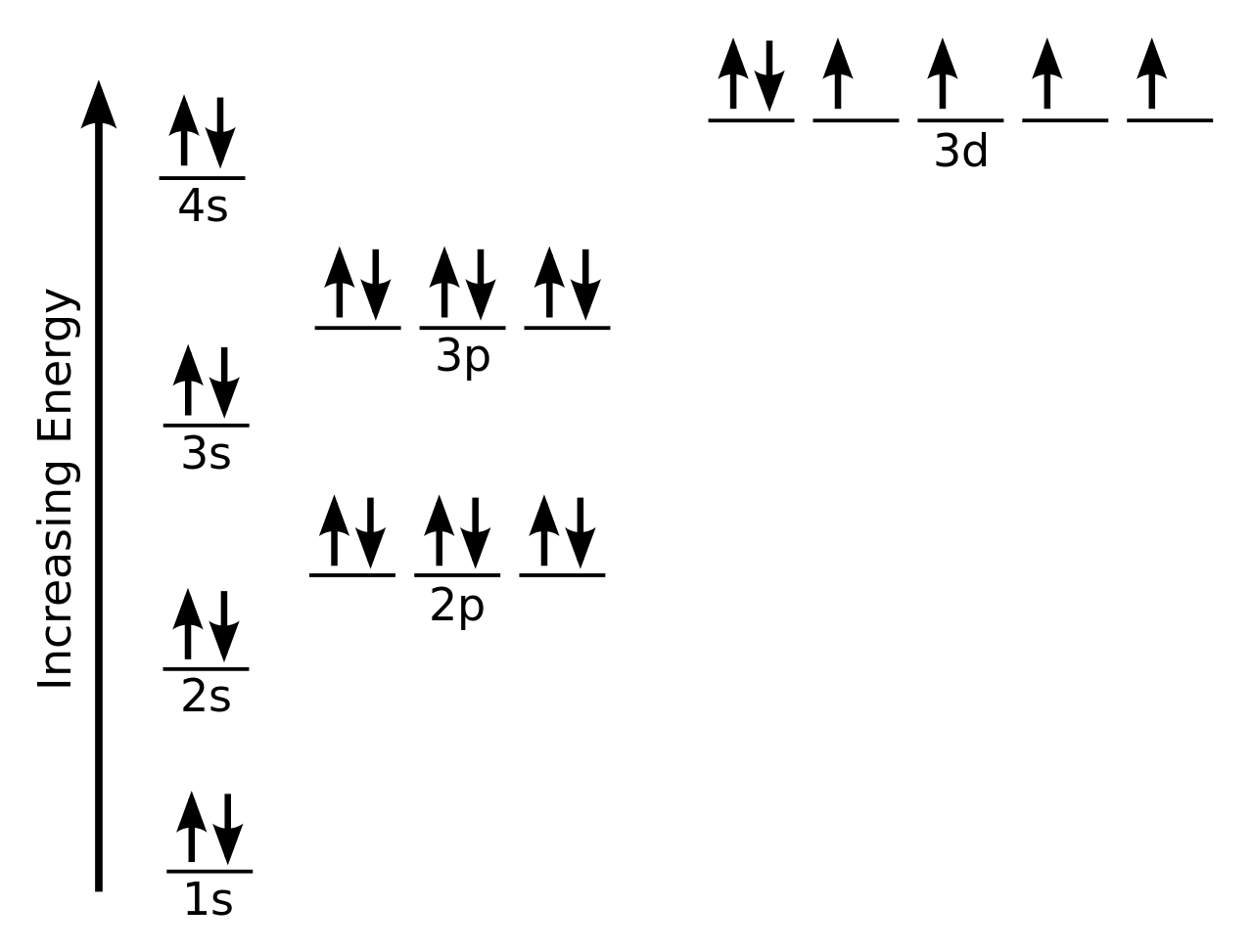
Orbital diagram of silicon. 14.12.2019 · In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ... 9.7 The orbital diagram below presents the final step in the CQ formation of hybrid orbitals by a silicon atom. (a) Do you think one or more electrons have been promoted? Why or why not? (b) What type of hybrid orbitals are being produced in this hybridization? [Section 9.5] 9.8 Consider the hydrocarbon drawn below. (a) What is the Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor.It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen ...
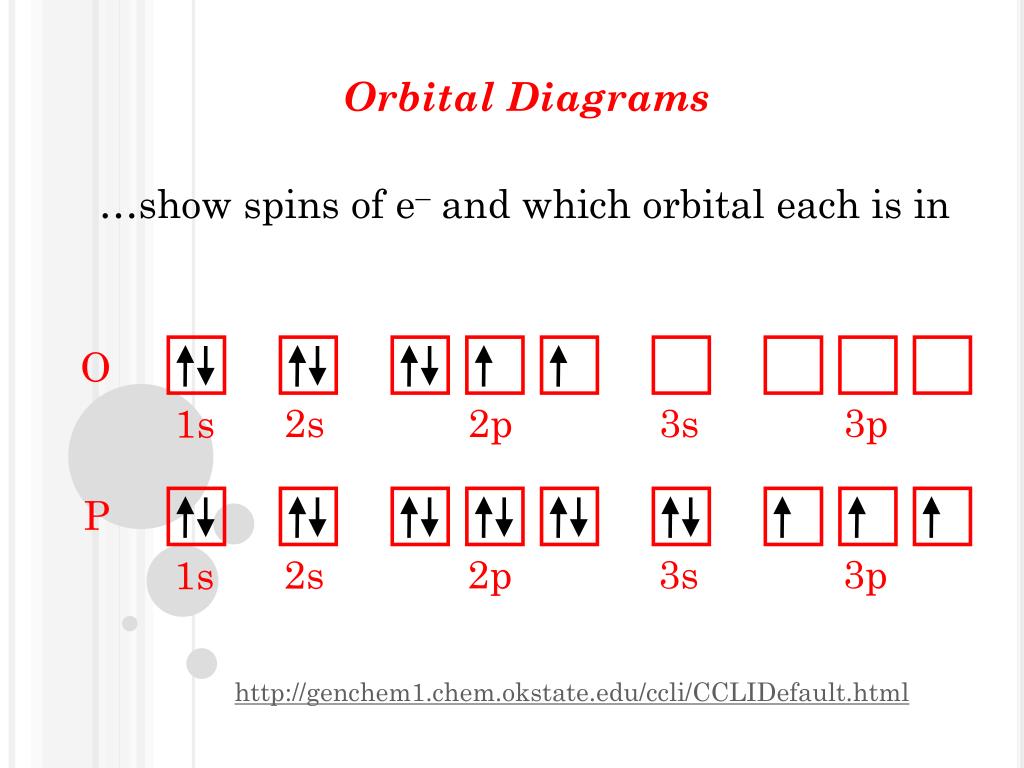
Orbital diagram. Silicon electron configuration ← Electronic configurations of elements . Si (Silicon) is an element with position number 14 in the ... 1s 2 2s 2 2p 6 3s 2 3p 2 Reduced electronic configuration Si: [Ne] 3s 2 3p 2. Below is the electronic diagram of the Silicon atom Distribution of electrons over energy levels in the Si atom 1 ... Si orbital Diagram. electron configuration of silicon si orbital diagram orbital diagram electron configuration and the noble gas notation for a silicon si atom electron configuration for silicon si how to write the electron configuration for silicon si since 1s can only hold two electrons the next 2 electrons for silicon go in the 2s orbital 31.10.2019 · Orbital filling diagram for silicon. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. Also the crystalline form is used in semiconductors. If you havent yet learned electron configurations you really need to go ahead. Commercial production depends on a reaction between sand sio2 and carbon at a. Silicon Orbital Diagram Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. That is one box contains 2 electrons. And for silicon, there will be 7 box representations for 14 electrons in a pair.
The Silicon Crystal and Conversion of Solar Energy to Electricity . Although the silicon atom has 14 electrons, their natural orbital arrangement allows only the outer four of these to be given to, accepted from, or shared with other atoms. The orbital diagram for an element shows the electron distribution of the electrons and the correct pairing of electrons with respect to electron spin. Orbital diagrams are pictorial descriptions of the electrons in an atom. In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Silicon atomic orbital and chemical bonding information. The p orbital can hold up to six electrons. Figure 12.21 The Molecular Orbital Energy-Level Diagram for a Linear Arrangement of n Atoms, Each of Which Contains a Singly Occupied s Orbital. This is the same diagram as Figure 9.35 "Bonding in Ozone", with the addition of the far right-hand portion, corresponding to n = 30 and n = ∞. As n becomes very large, the energy separation between adjacent levels becomes so small that a single ... When we write the configuration we'll put all 14 electrons in orbitals around the nucleus of the Silicon atom. In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six …
This time we have collected a handy of orbital diagrams with various types in high definition! There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagrams that you can save for free. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams.
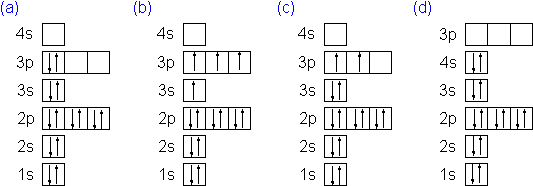
Question: examine the orbital diagram for the ground state electron configuration of silicon choose best dxplanataion for why this orbital diagram is incorrect foe the ground state of silicon. This problem has been solved! See the answer See the answer See the answer done loading.
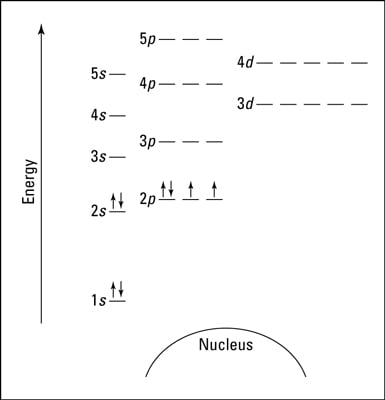
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
Above the 3p orbital those two arrows are both pointing up. 1s, 2s, 2p, 3s, and 3p represent orbitals. Now you have to imagine a line under the arrows (or the ones) the two ones above the 3p both...
Silicon") and Its Use the orbital diagram to find the third and eighth electrons. PROBLEM: Write a set of .Which ground-state atom has an electron configuration described by the following orbital diagram? A antimony B germanium C indium D lead E tin%(1).
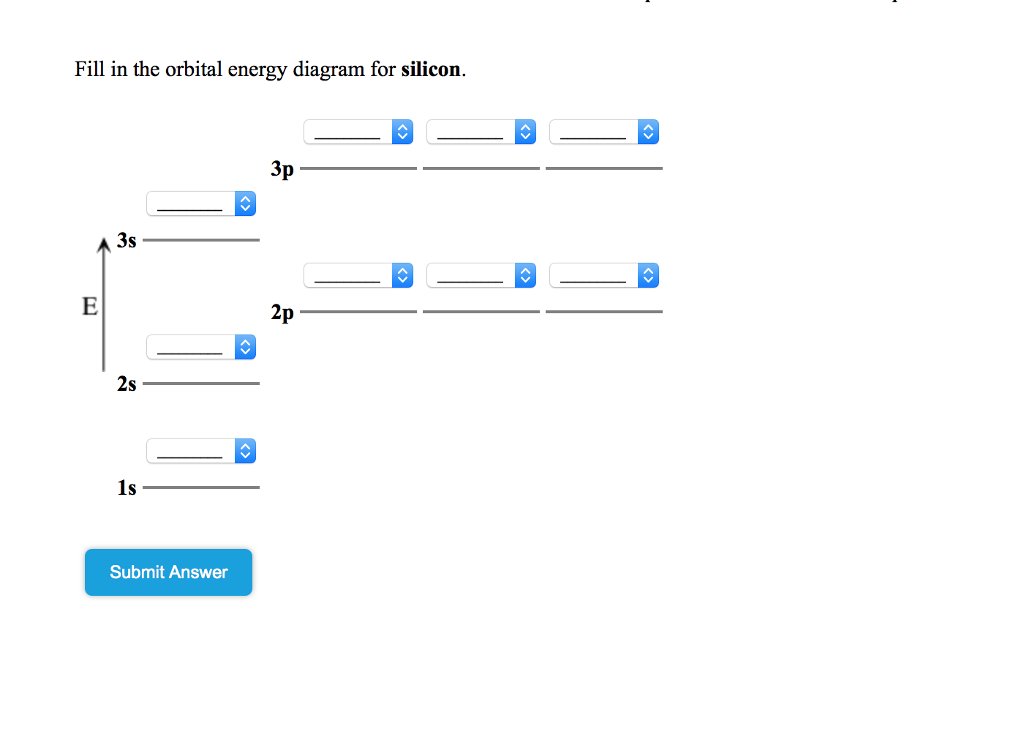
Orbital diagram for silicon (Si) Silicon (Si) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2. The valency of the element is determined by electron configuration in the excited state.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!
Oxidation States, ±4. Electrons Per Shell, 2 8 4. Electron Configuration, [Ne] 3s2 3p2. 1s2 2s2 2p6 3s2 3p2. Orbital Diagram.
03.10.2014 · Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom.
Silicon contains 14 electrons that are distributed among five energy levels. The orbitals 1s, 2s, 2p and 3s are filled first with 2, 2, 6 and 2 electrons, respectively. The remaining two electrons are placed in the 3p orbital.
The Orbital Diagram for Silicon: The orbital diagram for an element shows the electron distribution of the electrons, and the correct pairing of electrons with respect to electron spin.
01.11.2021 · Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10
Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2.The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
















0 Response to "37 orbital diagram of silicon"
Post a Comment