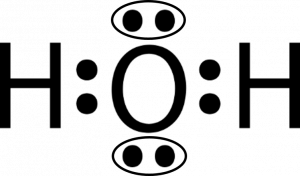
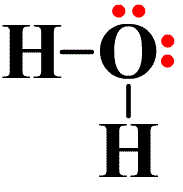
37 electron dot diagram for h2o
Jan 01, 2022 · Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure.
Orbital diagram for central atom after hybridization 5. Lewis Dot Diagram Worksheet - Worksheet Bunny Jul 14, 2021 · Lewis dot diagram worksheet use the bohr models to determine the number of valance electrons. Molecular Orbital Worksheet 1. Write the molecular orbital configuration of H 2 N 2 O 2 and F 2 molecules.

Electron dot diagram for h2o
Follow these steps to draw the lewis dot structure for H2S. Step 1: In the first step, determine the total valence electron present in H2S. As we know hydrogen only has one valence electron in its last shell and Sulfur belongs to the 16th group in the periodic table so it contains 6 electrons in its last shell. Dec 29, 2021 · Step 3: Sketch the Skeletal Diagram of the Molecule. In Lewis Structure, we use atomic symbols like C for carbon, H for hydrogen to represent the constituent atoms, and electron dot notation to represent the valence shell electrons. Let us look at the below skeletal sketch: The atomic symbols: Atomic symbols along with dot notations: So, according to the lewis dot structure of OF2, oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons. ∴ OF2 formula becomes AX 2 N 2 . According to the VSEPR chart, the molecule which has the AX 2 N 2 formula their molecular shape is bent and electron geometry is tetrahedral.
Electron dot diagram for h2o. Consider the two electron arrangements for neutral atoms A and B. What is the difference between atom A and atom B? A - 1s2, 2s2, 2p6, 3s1 B - 1s2, 2s2, 2p6, 5s1 Atom B has lost some inner electrons. The outer electron of atom B has moved to a lower energy state. The outer electron of atom B has moved to a higher energy state. So, according to the lewis dot structure of OF2, oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons. ∴ OF2 formula becomes AX 2 N 2 . According to the VSEPR chart, the molecule which has the AX 2 N 2 formula their molecular shape is bent and electron geometry is tetrahedral. Dec 29, 2021 · Step 3: Sketch the Skeletal Diagram of the Molecule. In Lewis Structure, we use atomic symbols like C for carbon, H for hydrogen to represent the constituent atoms, and electron dot notation to represent the valence shell electrons. Let us look at the below skeletal sketch: The atomic symbols: Atomic symbols along with dot notations: Follow these steps to draw the lewis dot structure for H2S. Step 1: In the first step, determine the total valence electron present in H2S. As we know hydrogen only has one valence electron in its last shell and Sulfur belongs to the 16th group in the periodic table so it contains 6 electrons in its last shell.














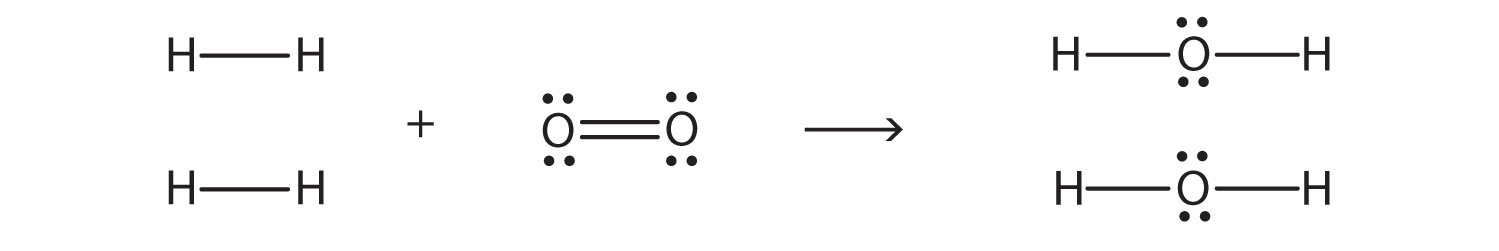
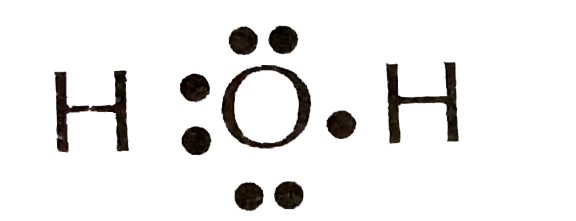


0 Response to "37 electron dot diagram for h2o"
Post a Comment