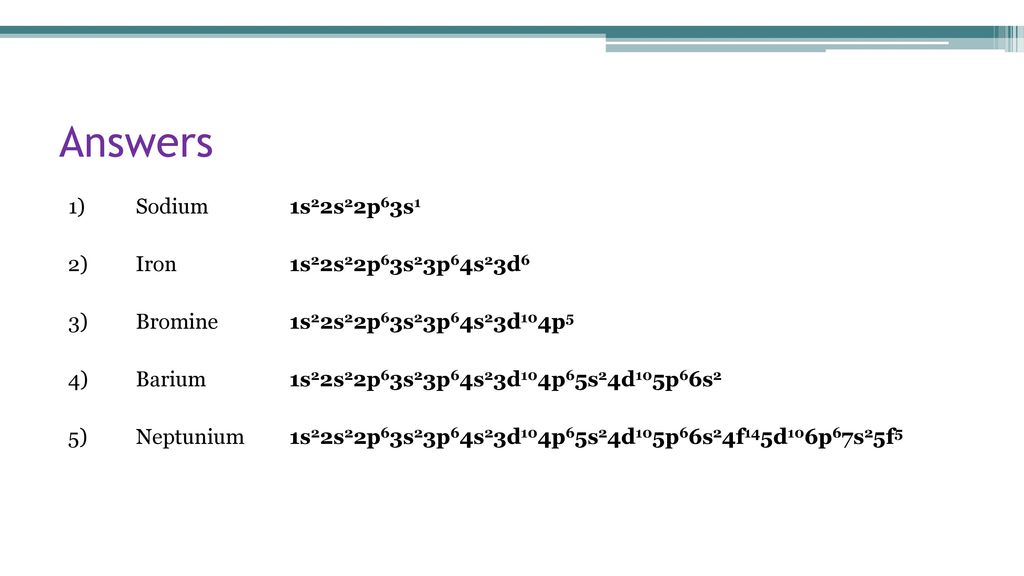
42 orbital diagram for barium
Chemistry Q&A Library Write the electron configuration, draw the orbital diagram, determine the distinguishing electron and determine the 4 quantum numbers for the distinguishing electron of the element Barium, Ba (write electron configurations as 1s2 2s2 2p6 3s2 3p6 with a space between each entry and no superscripts or [Rn] 7s2 5f4. write orbital diagrams with u for the up arrow, d for the ...
Barite, or barium sulfate (BaSO4), when ground is used as a filter for rubber, plastics, and resins. It is insoluable in water and so is used in X-rays of the digestive system. Barium nitrate, Ba (NO3)2, burns brilliant green and is used in fireworks. Learn more about the atomic number.
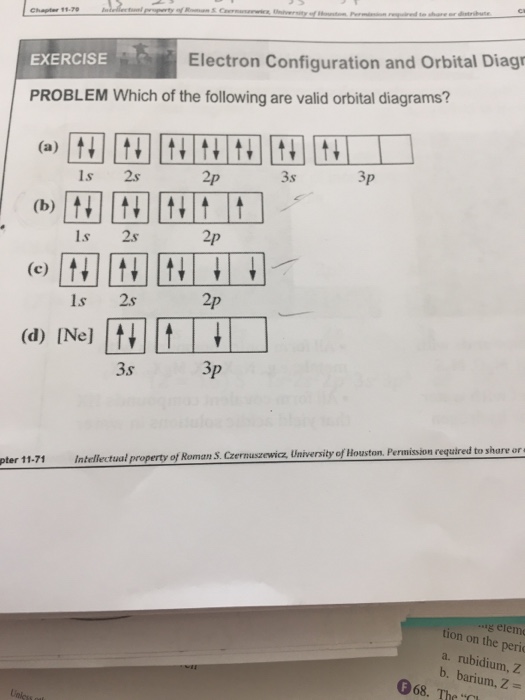
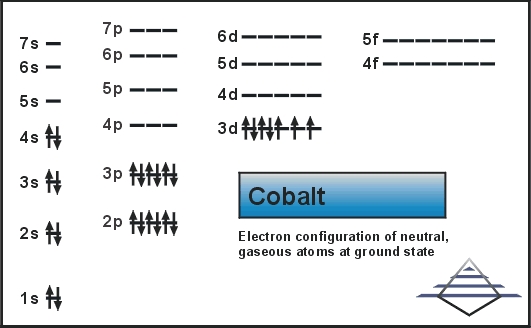
Exercise 7.3. 13. Hund's rule states that the most stable arrangement of electrons (for a ground state electron configuration) has a filled valence shell of electrons. has three electrons per orbital, each with identical spins. has values greater than or equal to +1. has the maximum number of unpaired electrons, all with the same spin.

Orbital diagram for barium
Orbital diagram of Tellurium (Te) 53: Orbital diagram of Iodine (I) 54: Orbital diagram of Xenon (Xe) 55: Orbital diagram of Caesium (Cs) 56: Orbital diagram of Barium (Ba) 57: Orbital diagram of Lanthanum: 58: Orbital diagram of Cerium (Ce) 59: Orbital diagram of Praseodymium (Pr) 60: Orbital diagram of Neodymium (Nd) 61: Orbital diagram of ...
Orbital Diagram For Barium 01.06.2019 6 Comments Step by Step: Electron Configurations and Electron Orbital Diagrams we need to count each box going from Hydrogen (#1) to Barium (#56), including Barium. The first two groups (columns) of the periodic table represent the 's' orbital group.
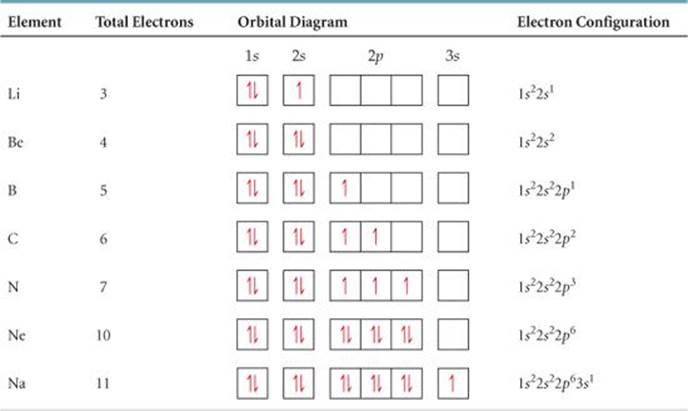
Looking at the periodic table, we need to count each box going from Hydrogen (#1) to Barium (#56), including Barium. H and He boxes: These boxes are in the 1st row, so 1. These boxes are in the "s" orbital region, so s. There are two boxes, so two electrons; the power is 2. 1s 2 Li and Be: These boxes are in the 2nd row, so 2.
Orbital diagram for barium.
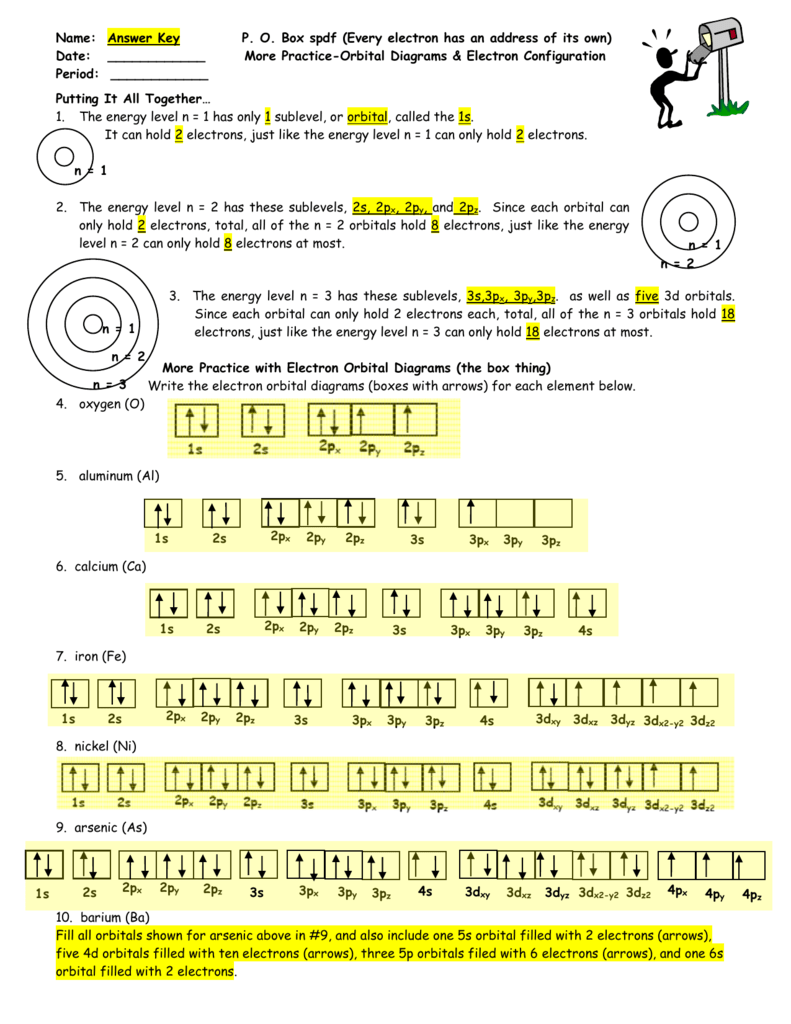
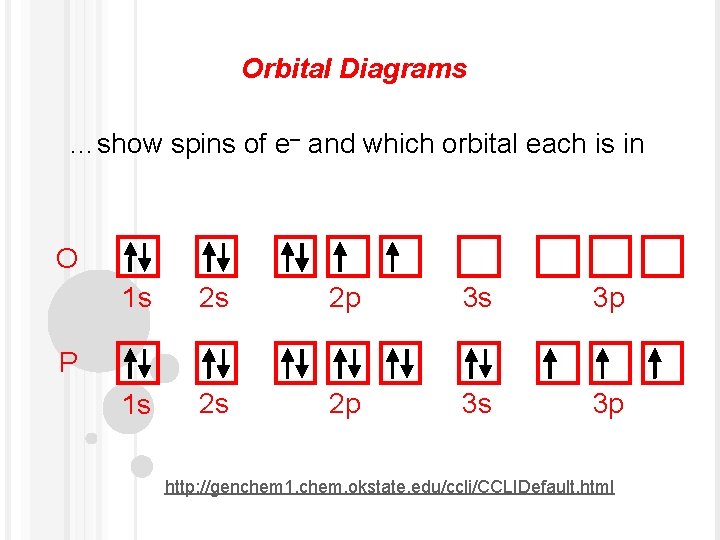
2. Electron Configuration (quicker to draw than orbital filling diagrams) 2 2 Ex. O 2 1s 2s 2p4 3. Electron Dot shows only the valence (outer energy level) electrons . . Ex. :Oxygen atom . O . 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram
Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium (atomic number: 56), an isotope of this . The first two groups (columns) of the periodic table represent the 's' orbital group.
Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1). Source: www ...
Download scientific diagram | Orbital elements for strong barium stars. No uncertainties are given for fixed parameters. The symbol > is used for uncertainties exceeding the parameter values in ...
Orbital Diagram For Barium The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium. Barium is an alkaline earth metal. This means that it is a group 2 element. Barium's atomic number is 56; this means that it has 56 protons in its nucleus and also.
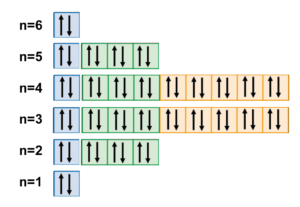
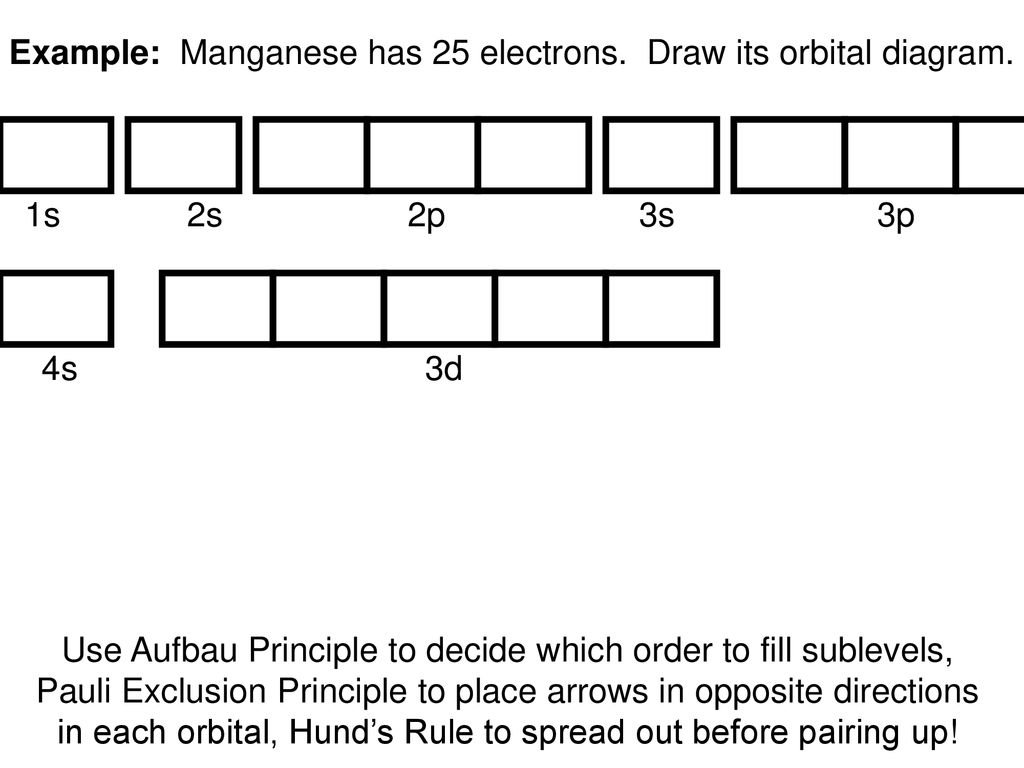
orbitals have one electron. Then additional electrons enter each orbital until 2 . electrons are in each orbital. Once all orbitals in a sublevel are filled (each with 2 . electrons), the next electron enters the next higher energy sublevel. The Aufbau diagram below illustrates the order of filling orbitals and sublevels.
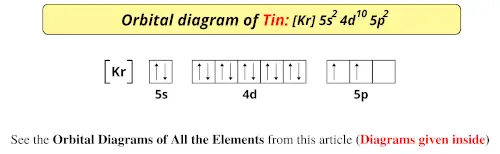
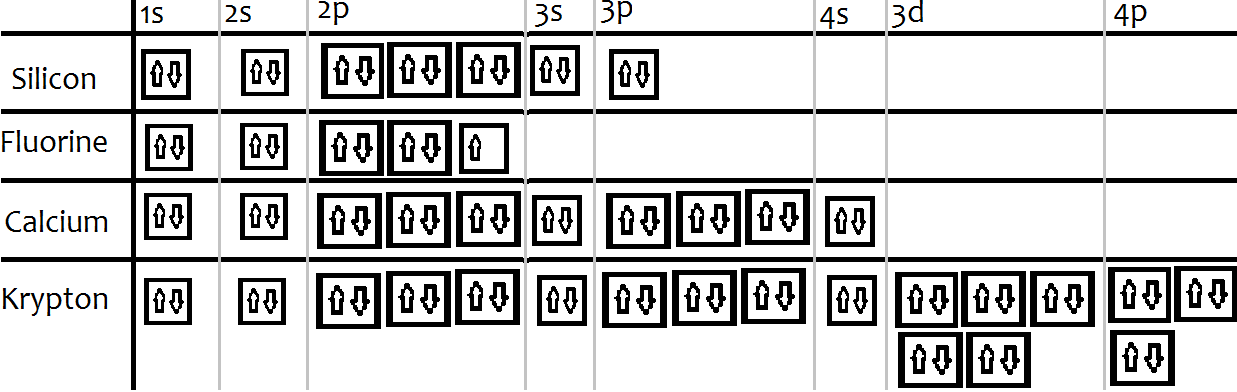
Orbital Diagram. 1s Soft, malleable, silvery-yellow metal. Uses Used in flares and fireworks for crimson color. Strontium is a long lived highly radioactive fallout product of atomic-bomb. The electron configuration for strontium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2, according to the Jefferson Lab website.
Context. A complete set of orbital parameters for barium stars, including the longest orbits, has recently been obtained thanks to a radial-velocity monitoring with the HERMES spectrograph installed on the Flemish Mercator telescope. Barium stars are supposed to belong to post-mass-transfer systems. Aims: In order to identify diagnostics distinguishing between pre- and post-mass-transfer ...
In order to write the I electron configuration we first need to know the number of electrons for the Barium (Ba) atom. There are 56 electrons for the Barium...
Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons.
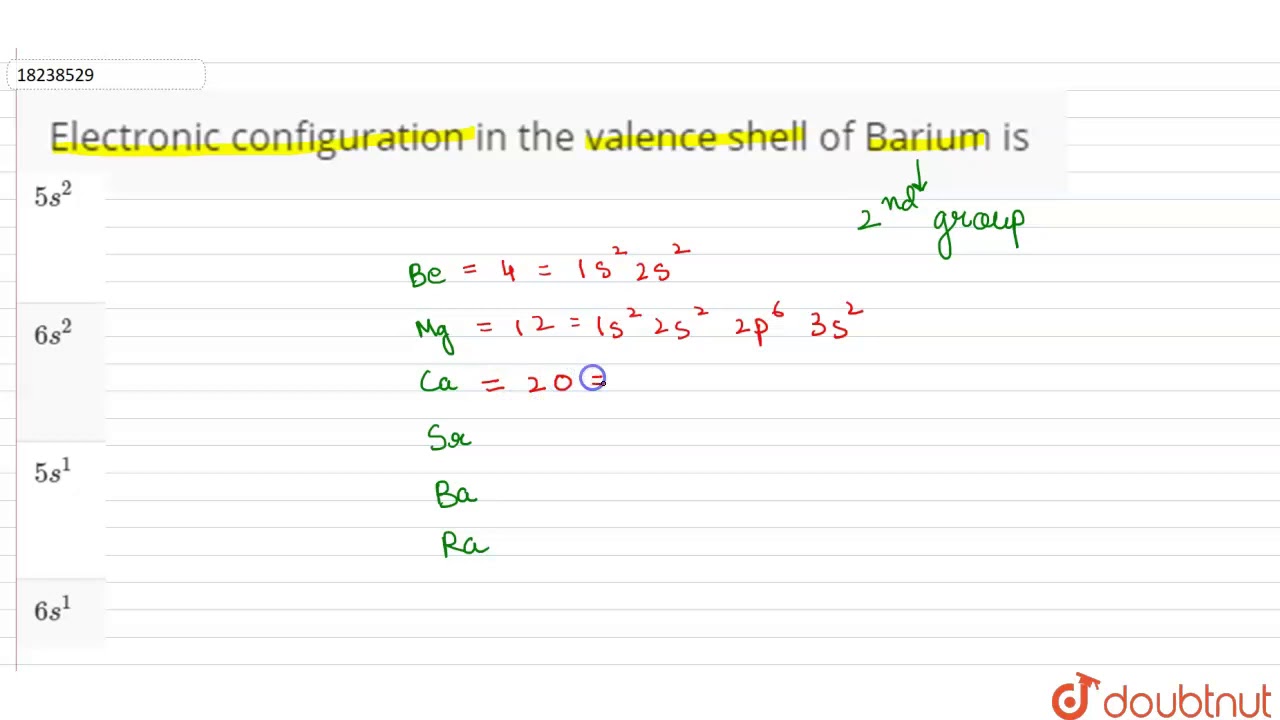
The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium must end with 6s2. The 6th row, s block, 2nd column. The full electron (s,p,d,f) configuration would be: 1s22s22p63s23p64s23d104p65s24d105p66s2
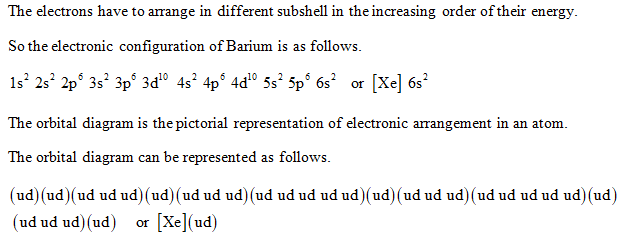
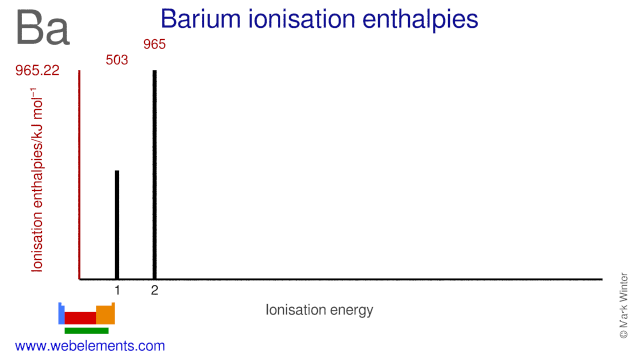
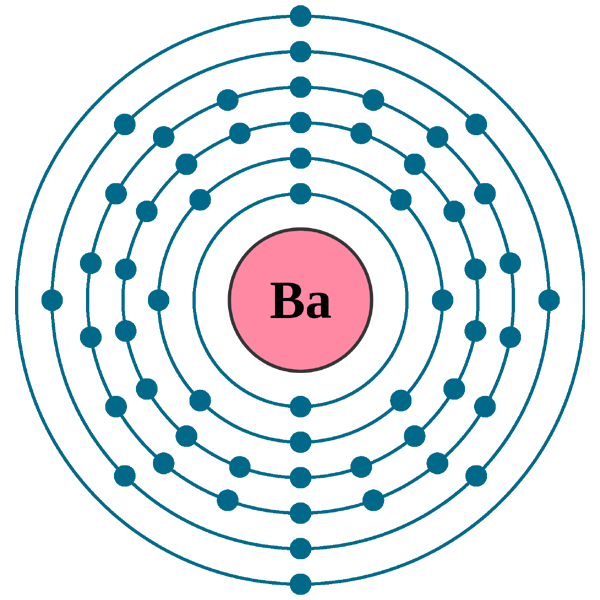
Binary compounds. Compound properties. Element reactions. Barium atoms have 56 electrons and the shell structure is 2.8.18.18.8.2. The ground state electron configuration of ground state gaseous neutral barium is [ Xe ]. 6s2 and the term symbol is 1S0. Schematic electronic configuration of barium. The Kossel shell structure of barium.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Barium is responsible for the green color that is observed in fireworks write the ground state ele 2 03047
Orbitals and Electron Configuration. The Periodic Table with Oxidation Numbers and Electron Configurations. S Block. P Block. D Block. F Block. 1. Hydrogen. 1.
Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium (atomic number: 56), an isotope of this .Barium is an alkaline earth metal. This means that the s,p,d,f electron configuration for Barium must end with 6s2. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1.
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿⇂ ↿⇂ ↿⇂ 4d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4f 5s ↿⇂ 5p ↿⇂ ↿⇂ ↿⇂ 5d 5f 6s ↿⇂ 6p 6d 6f ... Barium is an exception to the octet rule and will hold a maximum of four ...
Barium. Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. cesium ← barium → lanthanum.
Orbital Diagram. 1s ... Barite, or barium sulfate (BaSO4), when ground is used as a filter for rubber, plastics, and resins. It is insoluable in water and so is used in X-rays of the digestive system. Barium nitrate, Ba(NO3)2, burns brilliant green and is used in fireworks. Sources
Uses of Barium: Used in sparkplugs, vacuum tubes, fireworks, fluorescent lamps. Insoluble barium sulfate is used for body imaging. Additional Notes: Must be stored under kerosene to remain pure. Soluble barium salts are highly toxic. Barium Menu. Barium Page One. Overview of Barium; Barium's Name in Other Languages; Atomic Structure of Barium
Determine the # electrons in that orbital block for that element. count from left to right in that block until you get to the element; Find the noble gas (group 18) before the element. Begin filling the s block orbital of the next period and continue to fill until you get to the element.
Electron configuration ion barium. Full electron configuration of barium. Electron Affinity and Electronegativity of Barium Electron Affinity of Barium is 1395 kJmol. There are 56 electrons for the Barium. Three barium atoms each lose 2 electrons forming B a 2 and two nitrogen atoms each gain 3 electrons forming N 3 The resulted ionic compound.
barium 7. potassium 17. lead 8. aluminum 9. magnesium 10. sulfur . Dr. Mihelcic Honors Chemistry 2013-14 6 HW #5: Electron Configurations I. Write both the full electron configuration for each of the following elements ... Orbital Diagram Configuration Orbital Diagram Configuration . Dr. Mihelcic Honors Chemistry 2013-14 8 Homework Worksheet #7 ...
Electronic configuration of the Barium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 Electronic configuration of the Barium atom in ascending order of the levels:



![Electron Configuration | Chemistry [Master]](https://textimgs.s3.amazonaws.com/boundless-chemistry/agram-carbon-hund-27s-rule.svg)



















0 Response to "42 orbital diagram for barium"
Post a Comment