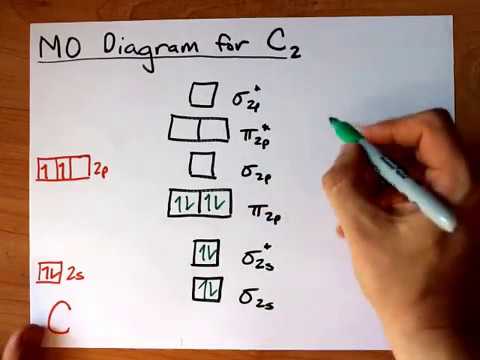
41 mo diagram of c2
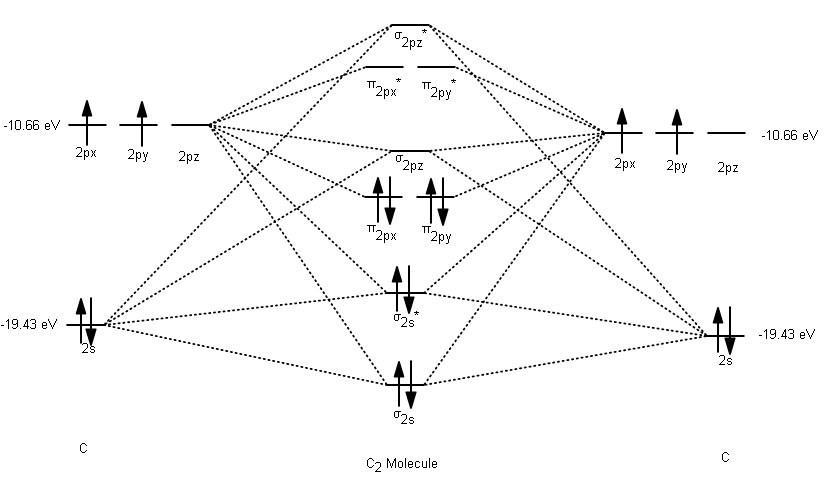
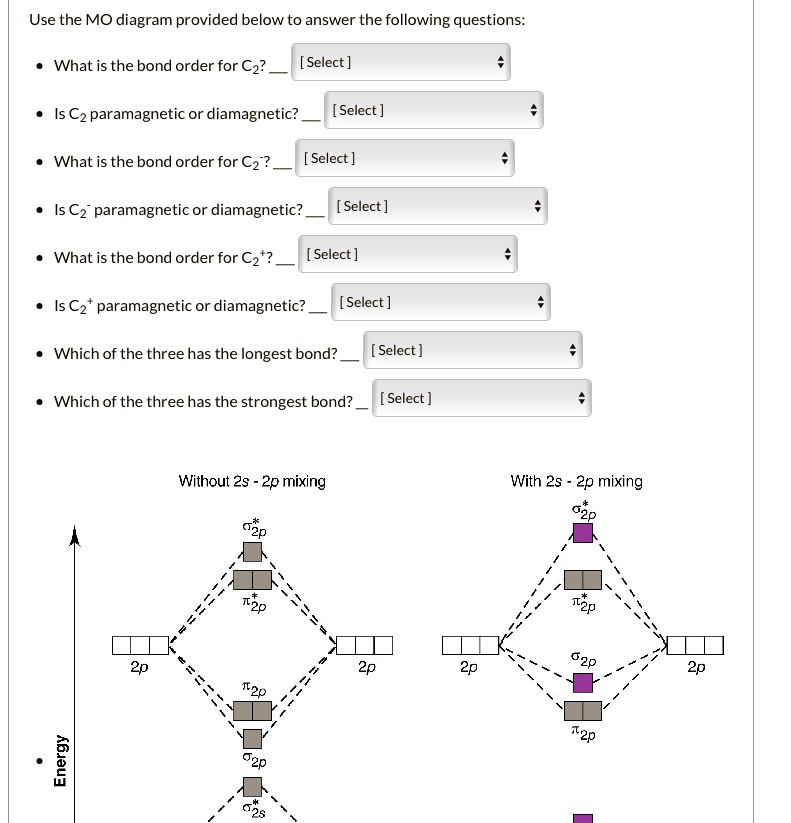
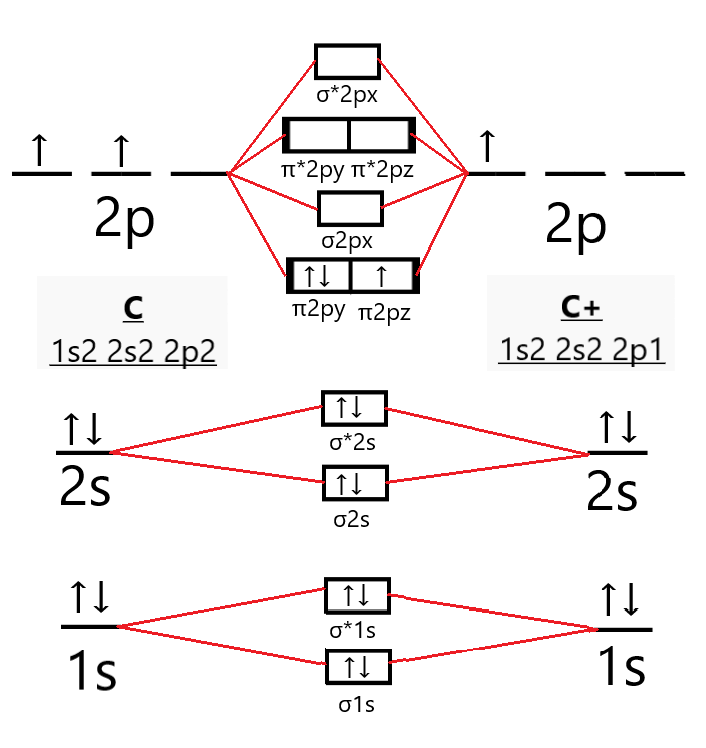
The answer is C2- because of bond orders. When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz orbital. As for bond orders it is 1/2* [ (#e- in bonding orbitals)- (#e- in antibonding orbitals)] Doing this, normally just C2 is 1/2* [ (8)-4]=2.
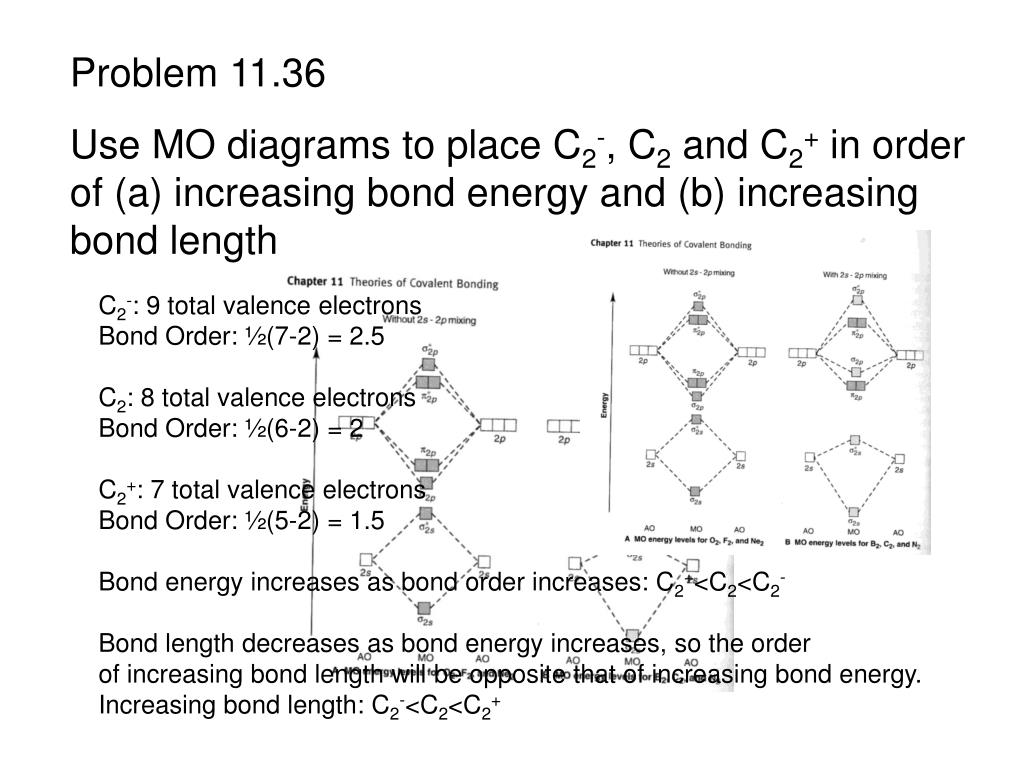
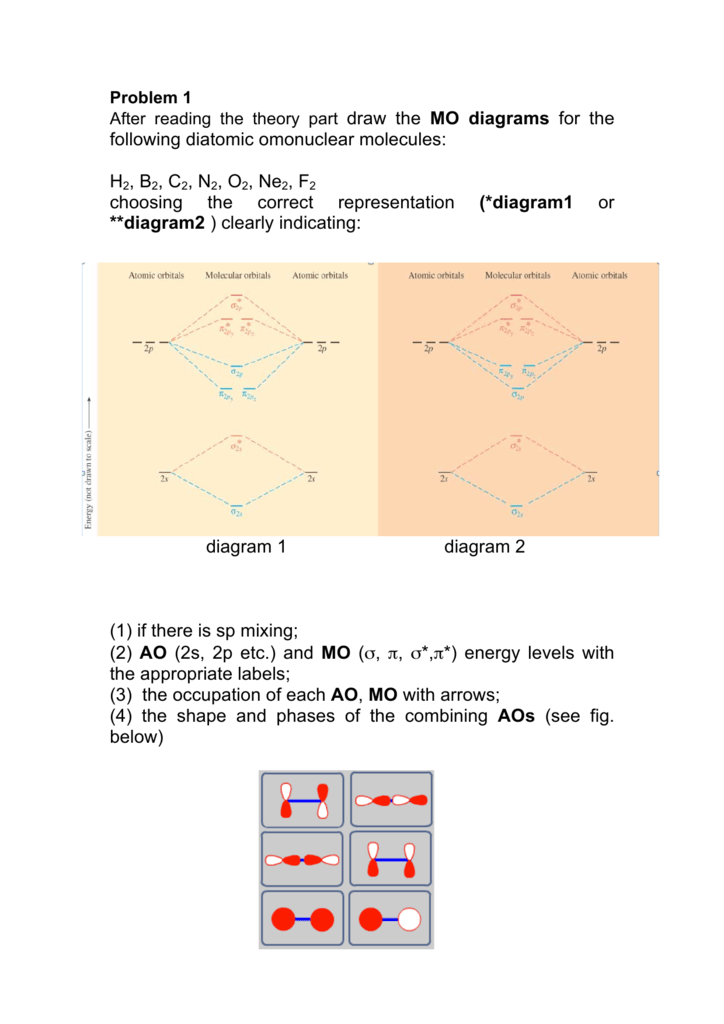
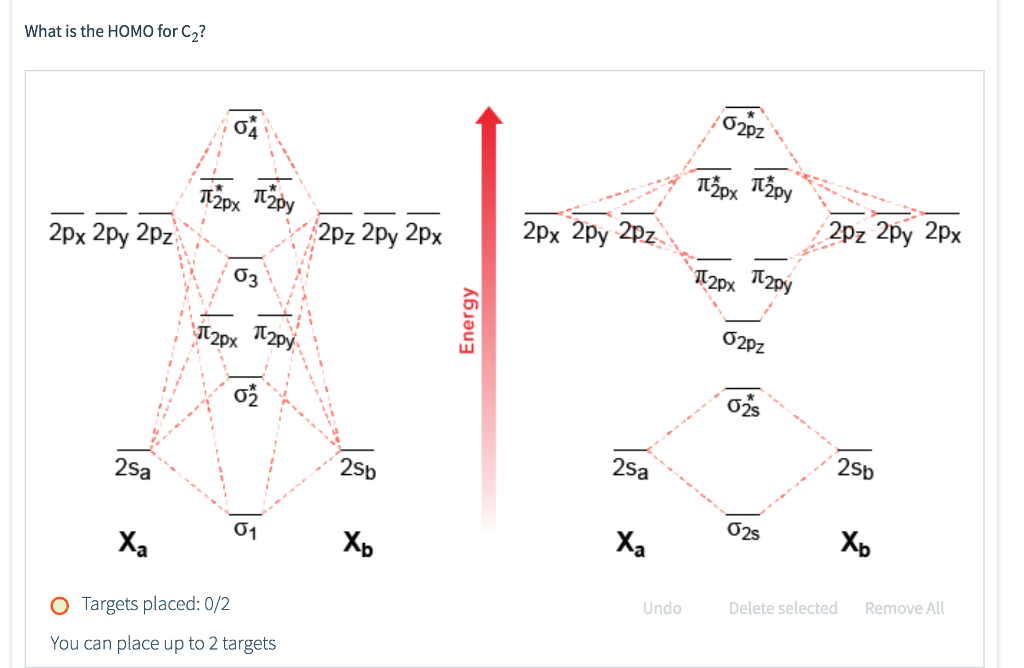
A) Draw the complete MO diagram for C2 - Assume NO sp mixing of orbitals when drawing the MO diagram. Compare this to the MO diagram of C2 with sp mixing. Calculate bond order for both Mo diagrams and designate if C, would be considered diamagnetic or paramagnetic in each case (MO diagram with sp mixing and MO diagram without sp mixing) B) Draw ...
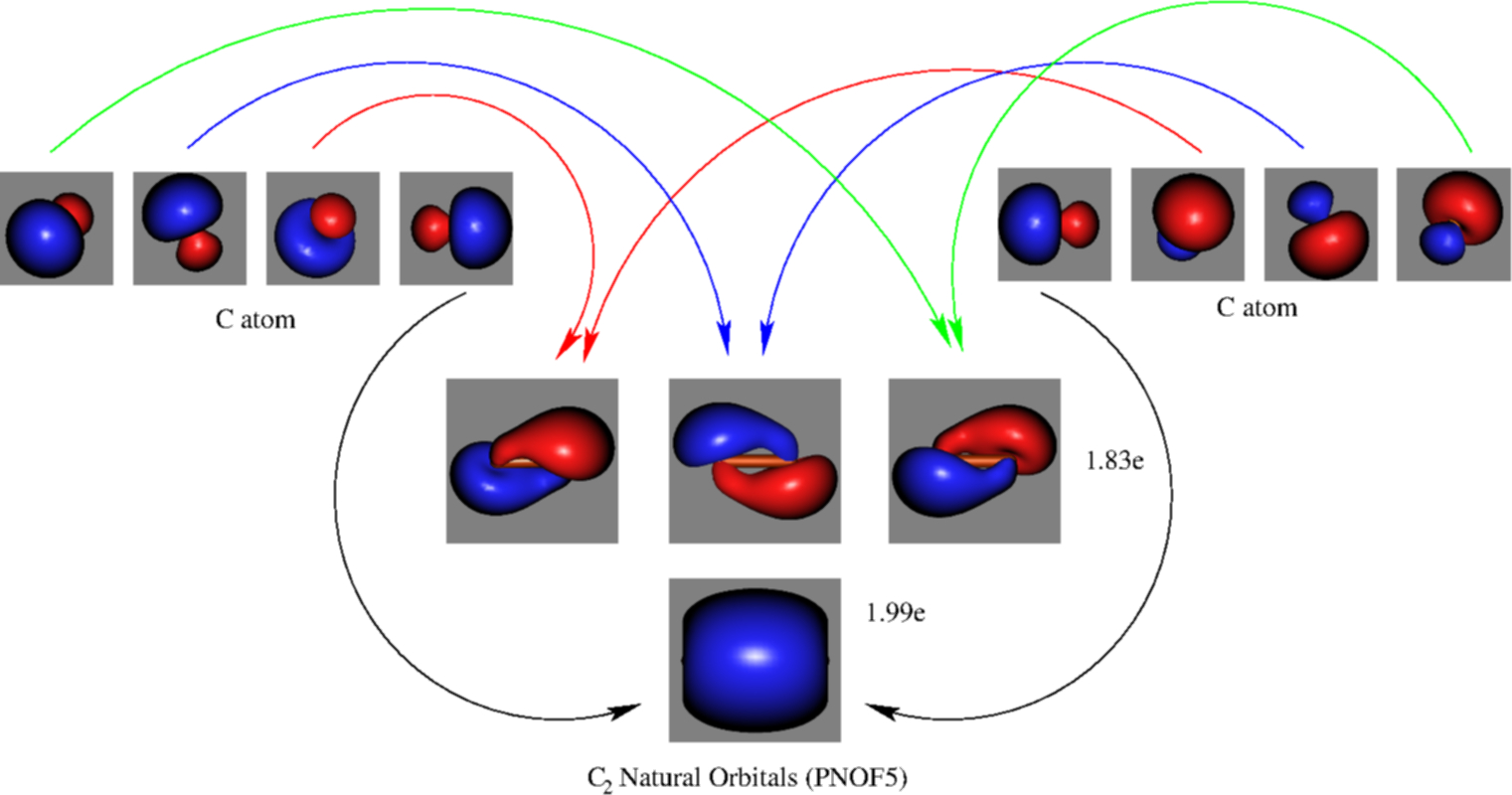
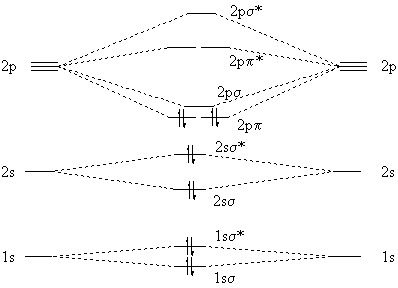
The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. C− 2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4(2pσ)1. Notice that because the extra electron is added to a bonding MO, the bond order of ...
Mo diagram of c2
Relative AO Energies for MO Diagrams H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p –19.4 eV –15.8 eV –32.4 eV –10.7 eV Photoelectron spectroscopy gives us a pretty good idea of the relative energies for AOs.
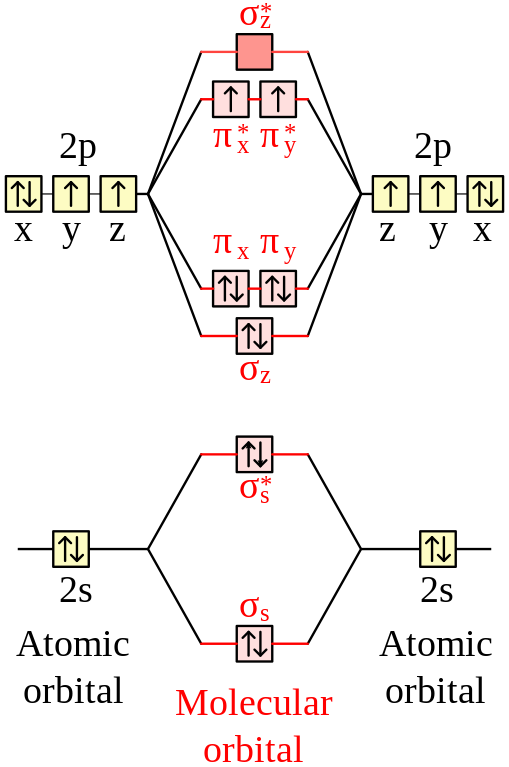
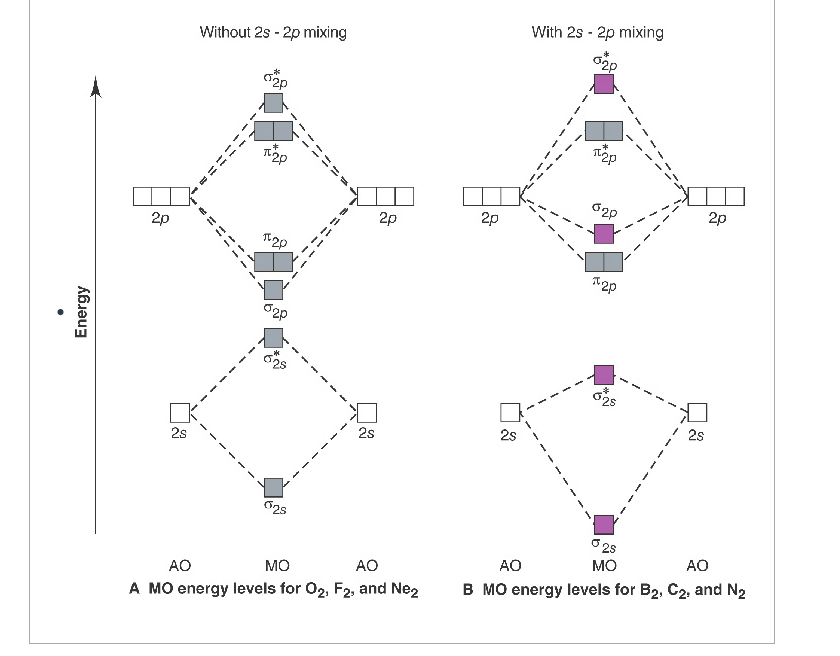
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
Answer to Solved Q1 : 1. Construct an MO diagram of C2- 2. Is C2-an sp 2 orbital from the carbon overlapping with a 2p orbital from each F atom to form sigma bonds, while another sp 2 hybrid orbital forms a sigma bond by overlaping with a sp 2 orbital for oxygen to form a sigma bond. A Pi bond is formed between C and O by the overlap between partially filled p orbitals overlapping between both ...
Mo diagram of c2.
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n (2px) 2 n (2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Molecular orbital diagram for c2 2-. The bond order of B2, C2, and N2 are 1, 2, and 3, respectively.
Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2.
#3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron.
Q. Using the molecular orbital model, write electronic configurations for the following diatomic species and calculate the bond orders.a. COb. CO+c. CO2+ ... Our tutors rated the difficulty of Use MO diagram to place C2-,C2 and C2+ in order of decreasin... as medium difficulty.





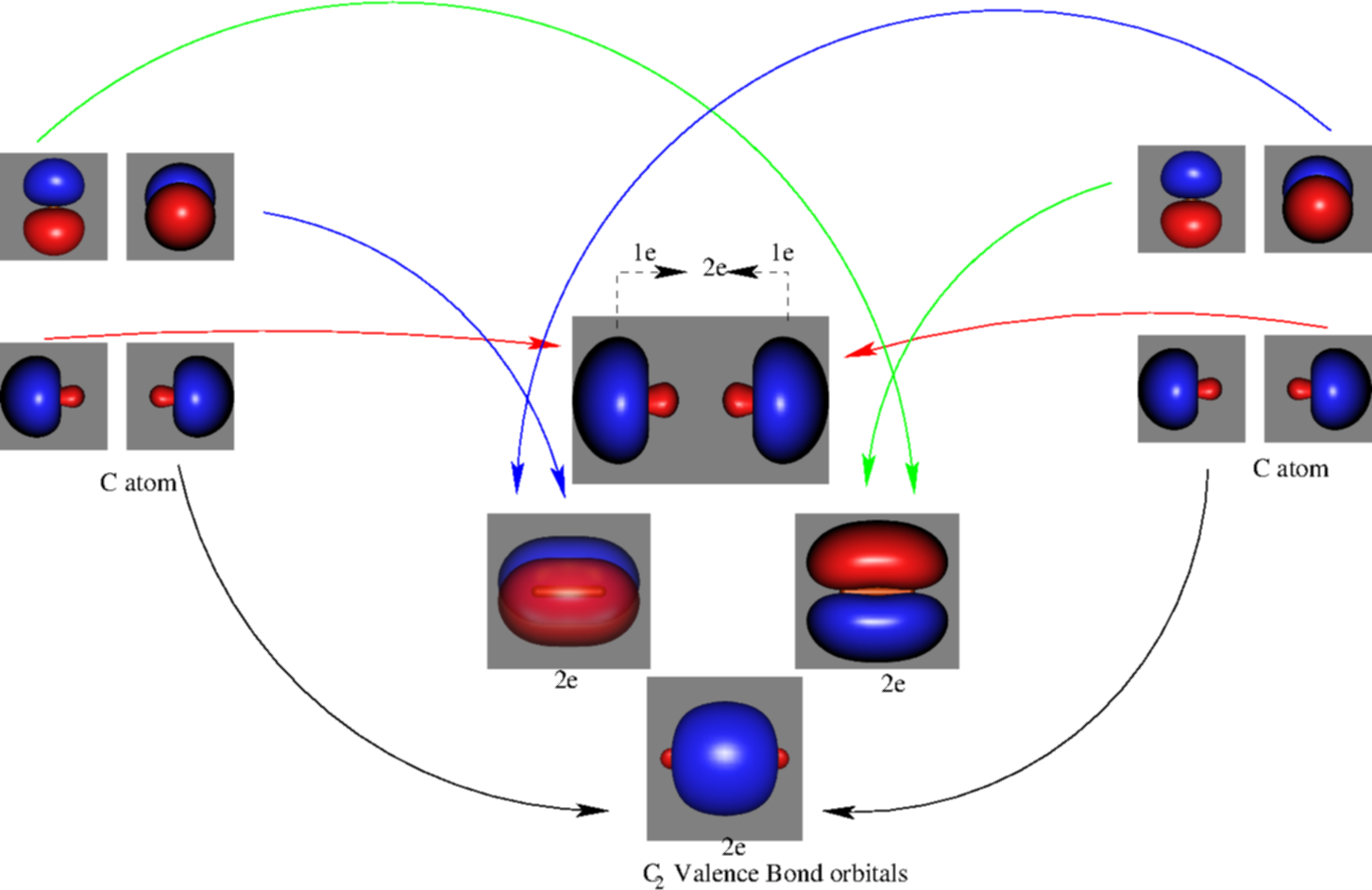












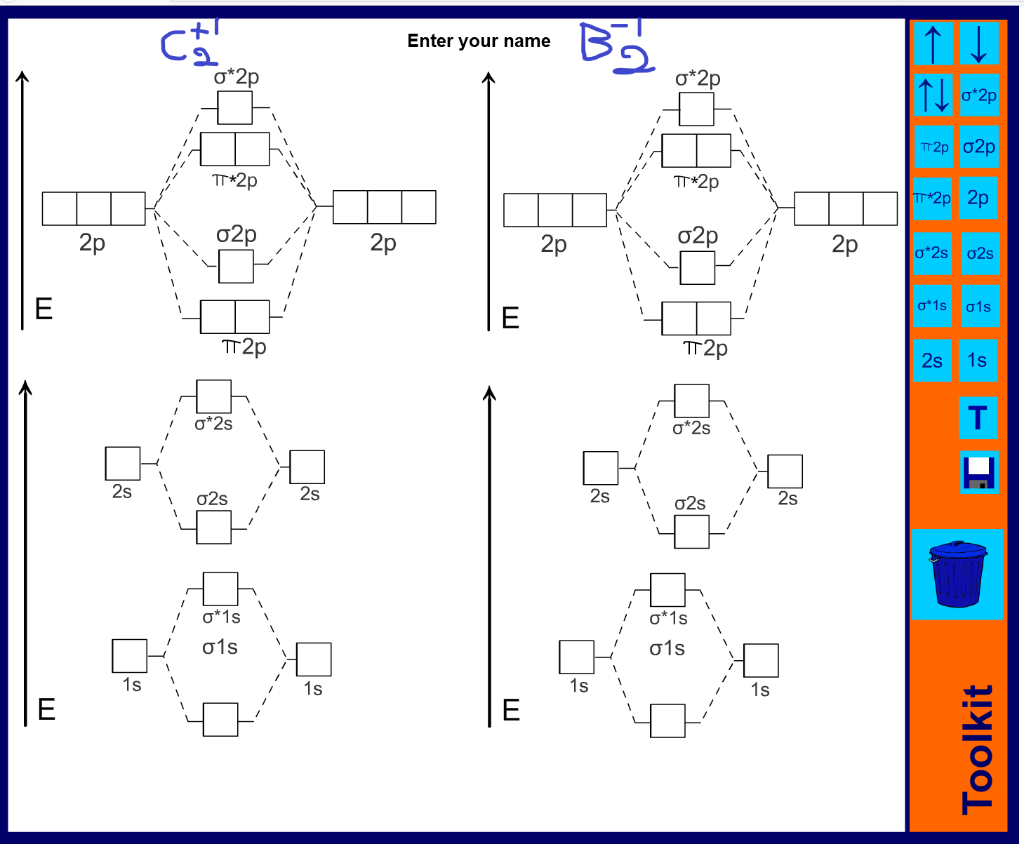
0 Response to "41 mo diagram of c2"
Post a Comment