40 how many electrons are depicted in the electron dot diagram
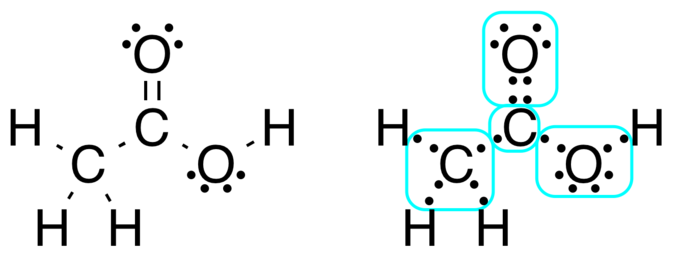
A Lewis dot structure is also called a Lewis structure a Lewis dot diagram an electron dot structure or a dot diagram. Lewis Dot Diagrams of Selected Elements. To draw the lewis dot structure of co2 we have to find out the valence electrons of carbon and oxygen firstwe express valence electrons as dots in lewis dot structure.
The electron dot diagram of elements or ions depict only the valence electrons. Carbon has atomic number of 6. The first shell has 2 electrons and gets filled. The second shell is filled by the remaining four electrons.The 4 electrons of the valence shell is shown in the electron dot diagram.
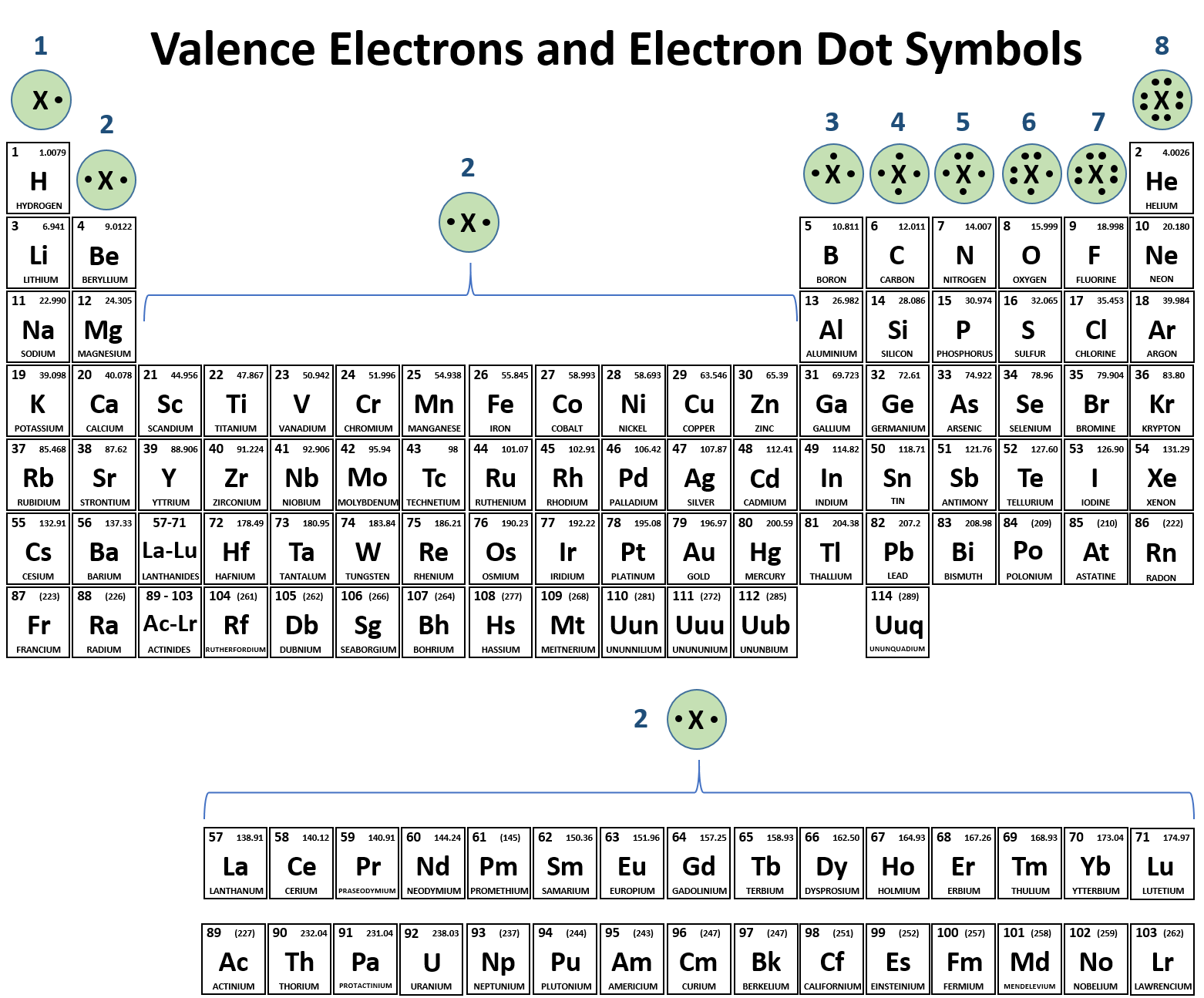
To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.

How many electrons are depicted in the electron dot diagram
To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s 2 2s 2 2p 6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
How to Draw a Lewis Dot Structure. Determine the total number of valence electrons to be depicted in the Lewis diagram. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine how many electrons must be added to central element. How many electrons are shown in the electron dot ...
number of protons = number of electrons. Hence, N atom has 7 electrons. - The electron configuration is 1s² 2s² 2p³. Hence, N atom has 2 + 3 = 5 valence electrons. So, five electrons are represented in electron dot diagram of N.
How many electrons are depicted in the electron dot diagram.
How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
Answer (1 of 3): Think about the nature of these elements: Carbon is unique in that it usually forms 4 bonds, while chlorine and fluoride are both halogens (usually forming 1 bond). Add the total valence electrons from each: 4 + 2(7) + 2(7) = 32 total ...
Electrons Answer Key. Electrons are found outside the nucleus. Valence electrons are the electrons in the outermost shell. The electron cloud is a visual model of the probable locations of electrons in an atom. Also to know, how many electrons do the elements in Group 2 have in their electron dot diagrams?
How many electrons are depicted in the electron dot diagram of an electrically neutral nitrogen atom? five. Which feature of a chemical equation represents the law of conservation of matter? ... 24 electrons, 20 neutrons 20 protons, 20 electrons, 24 neutrons 25 protons, 20 electrons, 24 neutrons
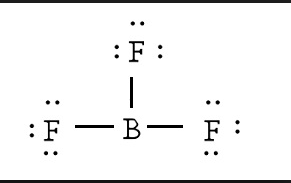
An electron 13, Electron dot diagram for boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;. Structure, properties, spectra, suppliers and links for: Boron nitride. Boron has 5 electrons. 3 are in the valence shell.
Correct answers: 3 question: How many electrons are depicted in the electron dot diagram of an electrically neutral oxygen atom? (only answer correctly or you will be reported) fivesixeighttwo
According to the periodic table, how many protons and electrons does an atom of argon (Ar) contain? answer choices. 18 protons, 40 electrons. 18 protons, 18 electrons. 20 protons, 18 electrons. 40 protons, 20 electrons. <p>18 protons, 40 electrons</p>. alternatives.
BHow many electrons should be shown in the Lewis dot structure for hydrogen. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Report an issue. 1 Nitrogen atom needs 3 electrons and all 3 Hydrogen atoms need 1 more electron to get stable.
How many electrons are depicted in the electron dot diagram of an electrically neutral carbon atom? 4 What feature of a chemical equation represents the law of conservation of matter?
Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices.
In the Lewis symbol, the electrons are depicted as two lone pair dots. What is the dot diagram? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence ...
How do you write a Lewis dot diagram? How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
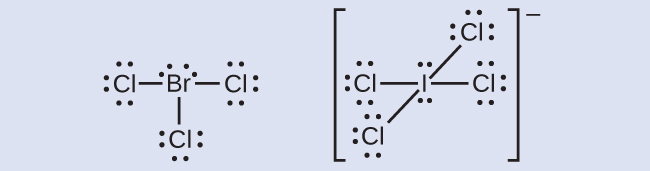
This diagram shows Lewis electron dot structures for a number of chemical species Click on a part of the image that represents a single pair of valence electrons shared between two atoms cI cI .Ci: Draw the Lewis structure for the ClO3 ion on paper How many valence electrons are found in the ion?
What is the Lewis dot diagram for Helium? The Lewis symbol for helium: Helium is one of the noble gases and contains a full valence shell. Unlike the other noble gases in Group 8, Helium only contains two valence electrons. In the Lewis symbol, the electrons are depicted as two lone pair dots.
The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons.
The Lewis Structure of CH2O is drawn as: 1. Search for the total already available valence electrons in a single formaldehyde CH2O molecule: It is twelve as two are coming from the two hydrogen atoms, four from the carbon atom, and six from the oxygen atom. 2. Search for how many more electrons are required to stabilize the octet of all the ...
A step-by-step explanation of how to draw the C2H4 Lewis Dot Structure (Ethene).For the C2H4 structure use the periodic table to find the total number of val...
In water the sharing is not equal. The oxygen atom attracts the electrons more strongly than the hydrogen. Hereof, what is the electron dot formula for water? Answer and Explanation: Water, or H2O H 2 O , has the electron dot structure shown below. The structure must have a total of 8 valence electrons because there are 2.
How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3.
Take for example nitrate ion, N O− 3. Nitrogen, Group V, has 5 valence electrons; oxygen, Group VI, has 6 valence electrons. And we throw in another electron, so that we have 5 +3 × 6 + 1 = 24 valence electrons, i.e. 12 electron pairs in the Lewis structure of N O− 3 to distribute around 4 centres. And thus we get O = N +( −O−)2.
How to Draw a Lewis Structure. Step 1: Find the Total Number of Valence Electrons. …. Step 2: Find the Number of Electrons Needed to Make the Atoms "Happy" …. Step 3: Determine the Number of Bonds in the Molecule. …. Step 4: Choose a Central Atom. …. Step 5: Draw a Skeletal Structure. …. Step 6: Place Electrons Around Outside Atoms.
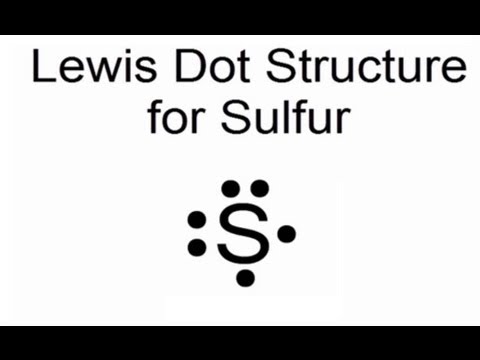
A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. As an example, an oxygen atom has six electrons in its outer shell. In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.
Valence Electrons and Lewis Dot Symbols. Mr. Causey explains valence electrons and how to use the periodic table to determine the valence electrons. Then Mr....
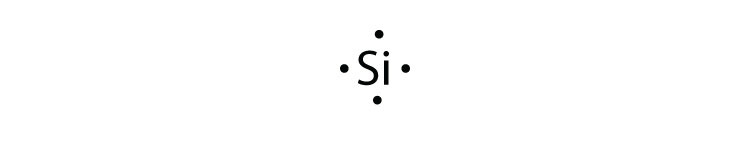


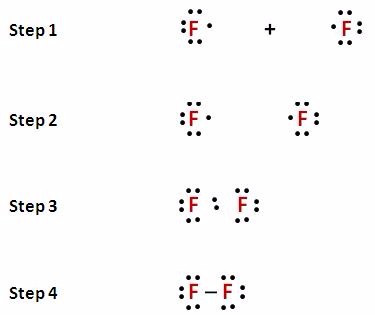
























0 Response to "40 how many electrons are depicted in the electron dot diagram"
Post a Comment