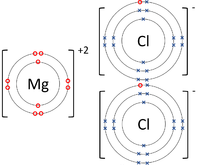
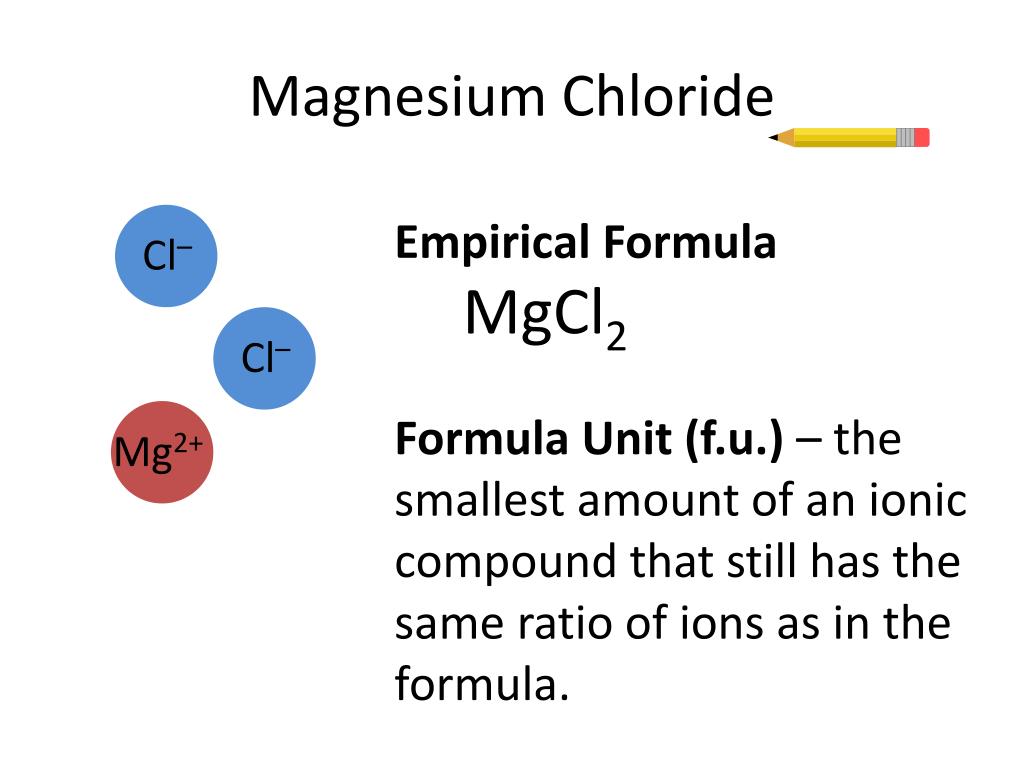
40 dot diagram of magnesium chloride
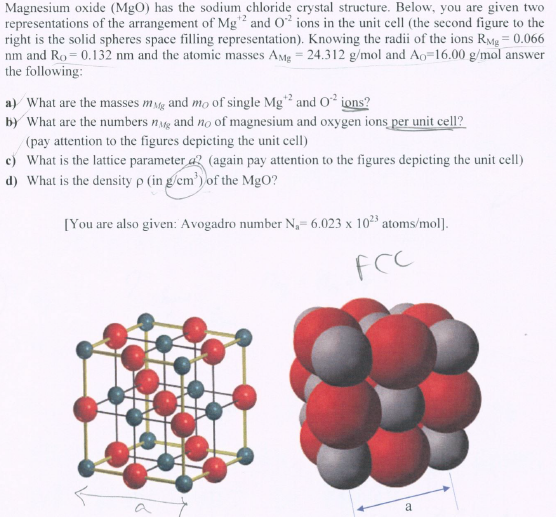
4 LE 2020 062004P23 (d) Magnesium reacts with chlorine to form magnesium chloride, MgCl 2 Magnesium chloride is an ionic compound. (i) Complete the dot-and-cross diagram in Fig. 2.1 of the ions in magnesium chloride. Show the charges on the ions. Mg..... Cl Cl Fig. 2.1 [3] (ii) One physical property typical of ionic compounds, such as MgCl 2, is that they are soluble Click here👆to get an answer to your question ️ Draw an electron dot diagram to show the formation of each of the following compounds:Magnesium Chloride. [H = 1, C = 6, Mg = 12, Cl = 17] .
Draw an electron dot diagram to show the formation of each of the following compounds magnesium chloride H 1 C 6 Mg 12 Cl 17.
Dot diagram of magnesium chloride
Visit http://ilectureonline.com for more math and science lectures!In this video I will show the Lewis structure for ionic compound for magnesiun chloride, M... Magnesium chloride is MgCl2, there should be two chlorine atoms like: Cl-Mg-Cl Magnesium loses an electron to each chlorine atom. Electron Dot Structure: Shell Diagrams For The First 20 Elements. Finding the electron dot structure of an. Magnesium: 12 electrons. Aluminium: 13 electrons. Magnesium chloride is another ionic compound. Unlike sodium chlorid, this compound does not have a 1:1 metal ion to nonmetal ion ratio. Lewis Electron Dot Symbols can be used to describe the ionic bonding in magnesium chloride. Lewis electron dot symbol to describe ionic bonding in magnesium chloride.
Dot diagram of magnesium chloride. See below. > "MgCl"_2 is an ionic compound. The electron configuration of "Mg" is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. "Mg" can get a noble gas s^2p^6 configuration by losing its two 4s electrons and forming a magnesium ion, "Mg"^(2+). The electron configuration of "Cl" is 1s^2 2s^2 2p^6 3s^2 3p^5. "Cl" can get a noble gas s^2p^6 configuration by gaining an electron and forming a chloride ion, "Cl"^"-". Magnesium chloride hexahydrate, BioUltra, for molecular biology, >=99.0% (KT) Magnesium chloride hexahydrate, BioReagent, suitable for cell culture, suitable for insect cell culture Magnesium chloride hexahydrate, puriss., meets analytical specification of Ph. Eur., BP, FCC, E511, 99-101%, <=0.0001% Al Example: Draw the dot- and- cross diagram of magnesium chloride. Step 1: Determine the type of bonding. As magnesium is a metal, and chlorine is a non- metal, magnesium chloride is an ionic compound. Step 2: Determine the charge of the metal and non-metal ion. Since magnesium is in group II, the charge is +2. give the a electron dot diagram of i magnesium chloride ii nitrogen iii methane b molecular structure of i magnesium chloride ii nitrogen iii methane - Chemistry - TopperLearning.com | vorl6bx44
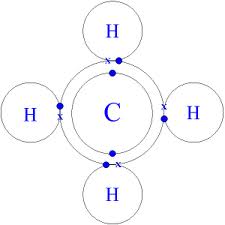
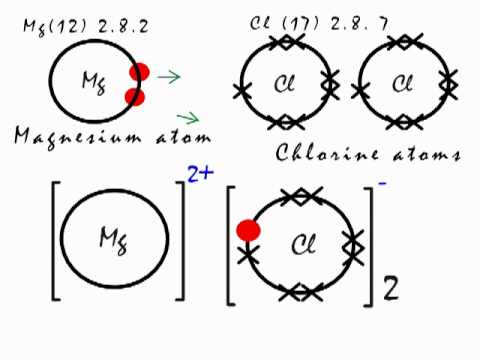
Use dot and cross to show electrons from Mg and Cl respectively, x x x x. Mg : + 2 [xClxx] > Mg2+ + 2[ xoClxx]^- > MgCl2 First, we have to draw the electron dot structure of magnesium and chlorine, to draw electron dot structure we have to write the electron configuration of magnesium and chlorine. Magnesium has atomic number 12, so there are 12 electrons in magnesium atom. Its electronic configuration will be: $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}$ Magnesium Chloride (MgCl2) - Magnesium Chloride is the chemical name of MgCl2. Visit BYJU'S to understand the properties, structure and uses of Magnesium Chloride (MgCl2) explained by India's best teachers. On the other hand Cl has even electrons in its atom so it gains one electron to acquire inert gas configuration and forms negatively charged ion or an anion. (a) Show the formation of magnesium chloride and sodium chloride by transfer of electrons. (b) Identify the ions present in these compounds.
What is the dot structure of MgCl2? Lewis Structure of MgCl2 Mg has 2 valence electrons whereas Cl has 7 valence electrons. The total number of valence electrons in a molecule of magnesium chloride = 2*1 + 7*2 = 16. Magnesium, as we all know, is an alkaline earth metal. Chlorine, on the other hand, is a halogen and therefore a non-metal. Dot diagram of magnesium chloride. The formation of magnesium chloride can be thought of as a reaction involving magnesium metal mg and chlorine gas cl2. There are two types of diagrams one is the lewis diagram the other is the electron dot diagram. Ionic bonding chemistry for non majors. MgCl2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. MgCl2 is an ionic halide salt consisting of magnesium and chlorine elements. It is inorganic in nature that may appear as a white or colorless crystalline solid compound. In its anhydrous form, it consists of about 25.5% Mg by mass and has a molar mass of about 95.211 g/mol. Structure of Magnesium Chloride. Magnesium chloride has a crystalline structure. The positively charged magnesium ion - Mg +2 and negatively charged chlorine ions Cl-create the ionic bond by attracting each other. And, magnesium chloride formation occurs by transferring electrons of the outer orbits.
(b) Chlorine forms compounds with magnesium and with carbon. (i) Draw a dot and cross diagram to show the electronic structure of the compound magnesium chloride (only the outer electrons need be shown). Include the charges present. (2) (ii) Draw a dot and cross diagram to show the electronic structure of the compound
For MgCl2 we have an ionic compound and we need to take that into account when we draw the Lewis Structure. We'll first draw the metal and ...
In this video, we will focus on dot and cross drawing of magnesium chloride ionic compound, MgCl2. The Periodic Table.
Draw an electron dot diagram to show the formation of each of the following compounds: (i) Methane (ii) Magnesium Chloride asked Jan 30, 2019 in Chemistry by Amoli ( 50.1k points) icse
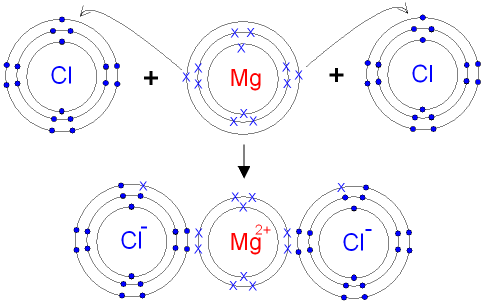
One magnesium atom loses two electrons, to become a +2 ion (cation).Two chlorine atoms gain one electron each to become two -1 ions (anions).These are held t...
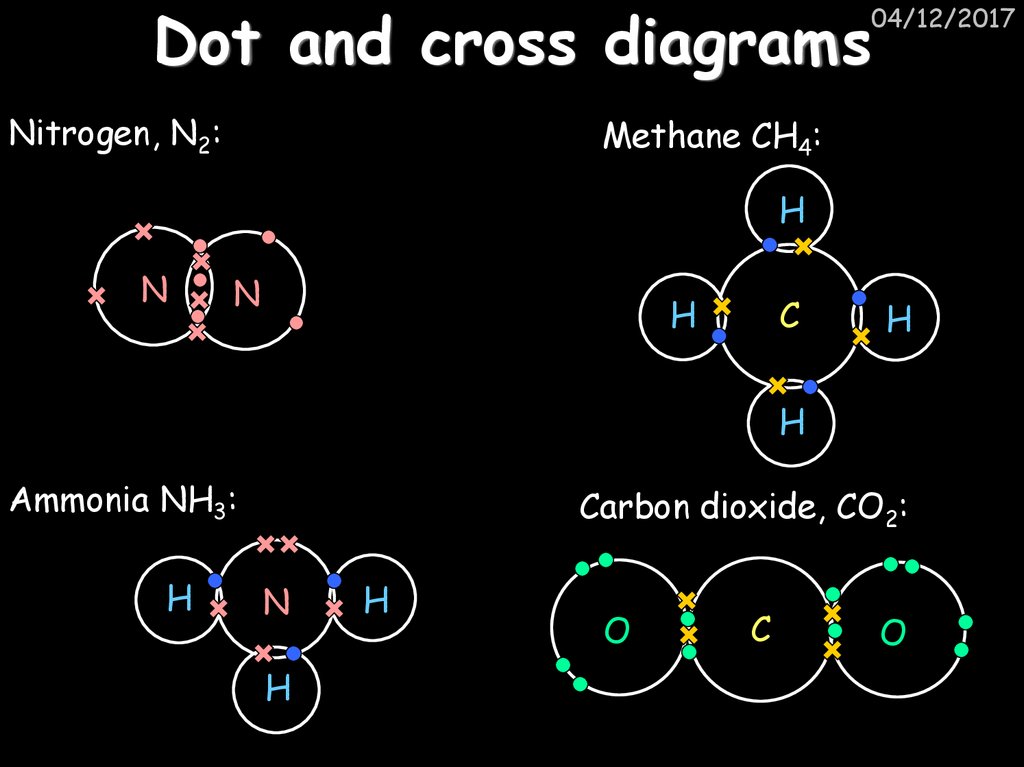
Here are some other examples of dot and cross diagrams for the formation of ions in some ionic compounds - magnesium oxide and calcium chloride. 1. Ionic bonding in magnesium oxide
Magnesium chloride is a compound which is composed of positive and negative ions. A covalent bond in which electrons are shared equally is called a what? It is called a nonpolar bond. What is an element? ... An electron dot diagram shows an atom's number of valence electrons.
what is the electron dot diagram for magnesium oxide well magnesium oxide is an ionic species which we could represent as mg 2 o 2 elemental magnesium has 12 nuclear protons z=12 it has 2 valence electrons that are conceived to be lost when it undergoes oxidation to mg 2 mgrarrmg 2 2e i elemental atomic oxygen has 8 electrons z=8. Download.
Atomic Structure. The Ionic Bond formation for Magnesium Chloride.. Magnesium is in group 2 of the periodic table. A magnesium atom will lose 2 electrons to form a stable 2 + ion.. Chlorine is in group 7 of the periodic table. A chlorine atom will gain 1 electron to form a stable 1-ion.. In this example the electrons are shown as dots and crosses.. Two chlorine atoms will each gain one ...
The slideshow shows dot and cross diagrams for the ions in sodium chloride, magnesium oxide and calcium chloride. 1. Ionic bonding in sodium chloride Question. Draw a diagram, with outer electrons ...
The electron-dot structure of MgCl2 is: The magnesium atom transfers its one electron to each chlorine atom to form an ionic bond.
- draw dot and cross diagrams for cations and anions with electronic configurations ... Chlorine forms compounds with magnesium and with carbon. i) draw a dot and cross diagram to show the electronic structure of the compound magnesium chloride (only the outer electrons need to be shown). Include the charges present.
Magnesium chloride | MgCl2 or Cl2Mg | CID - structure, chemical names, physical and chemical properties, classification, patents, literature, biological. Refer to the video. You need to be able to draw dot-and-cross diagrams to show the ions in some common ionic The result is a sodium ion (2,8)+ and a chloride ion (2,8,8)-.
Dot-and- cross diagram of magnesium chloride Intermolecular Force, ... Drawing dot- and- cross diagrams, showing the electrons in the outermost shells.
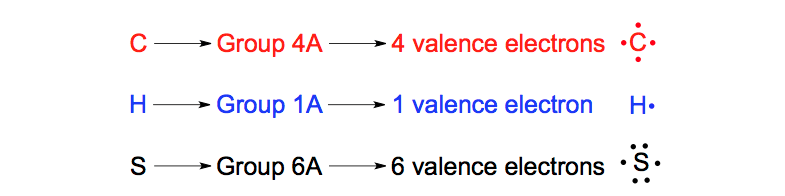
Quiz - Drawing Bohr and Electron Dot (or Lewis Dot) Diagrams A concise 1-page quiz worth 27 points that provides a review of 4 connected topics: element math (calculating the # of protons, electrons, and neutrons), drawing Bohr diagrams, drawing Lewis or electron dot diagrams, and analyzing how the electron structure of elements changes moving ...
The Lewis dot structure for Magnesium is an Mg with 2 dots which stand for its two valence electrons. ... Similarly one may ask, what is the electron dot structure of magnesium chloride? MgCl2 is an ionic compound. The electron configuration of Mg is 1s22s22p63s23p64s2 .
Lewis dot diagram for magnesium chloride. Posted on march 29 2019 by admin. Cl can get a noble gas s2p6 configuration by gaining an electron and forming a chloride ion cl. Electron Dot Diagram For Magnesium Bonding Basics Bromide Dot Diagram Wiring Diagram Data Schema Write The Electron Dot Structures For Sodium 11 Oxygen 8 ...
Magnesium chloride is another ionic compound. Unlike sodium chlorid, this compound does not have a 1:1 metal ion to nonmetal ion ratio. Lewis Electron Dot Symbols can be used to describe the ionic bonding in magnesium chloride. Lewis electron dot symbol to describe ionic bonding in magnesium chloride.
Magnesium chloride is MgCl2, there should be two chlorine atoms like: Cl-Mg-Cl Magnesium loses an electron to each chlorine atom. Electron Dot Structure: Shell Diagrams For The First 20 Elements. Finding the electron dot structure of an. Magnesium: 12 electrons. Aluminium: 13 electrons.
Visit http://ilectureonline.com for more math and science lectures!In this video I will show the Lewis structure for ionic compound for magnesiun chloride, M...



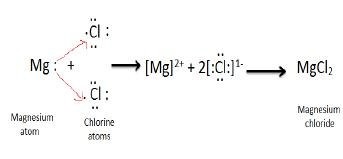






















0 Response to "40 dot diagram of magnesium chloride"
Post a Comment