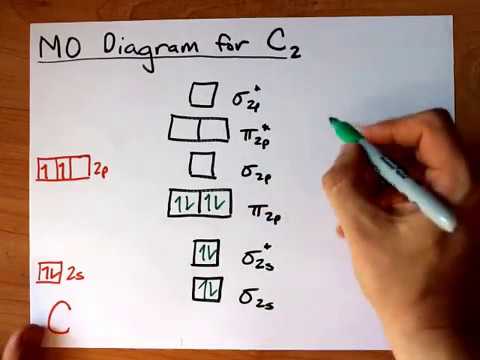
38 c2 molecular orbital diagram
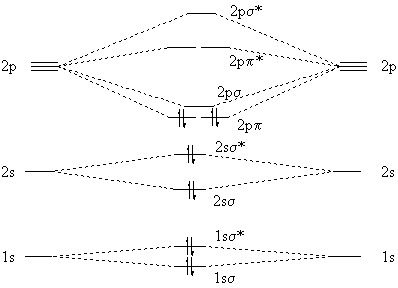
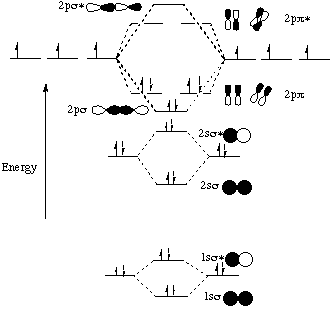
04.09.2021 · The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure \(\PageIndex{7}\)). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals …
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to ...
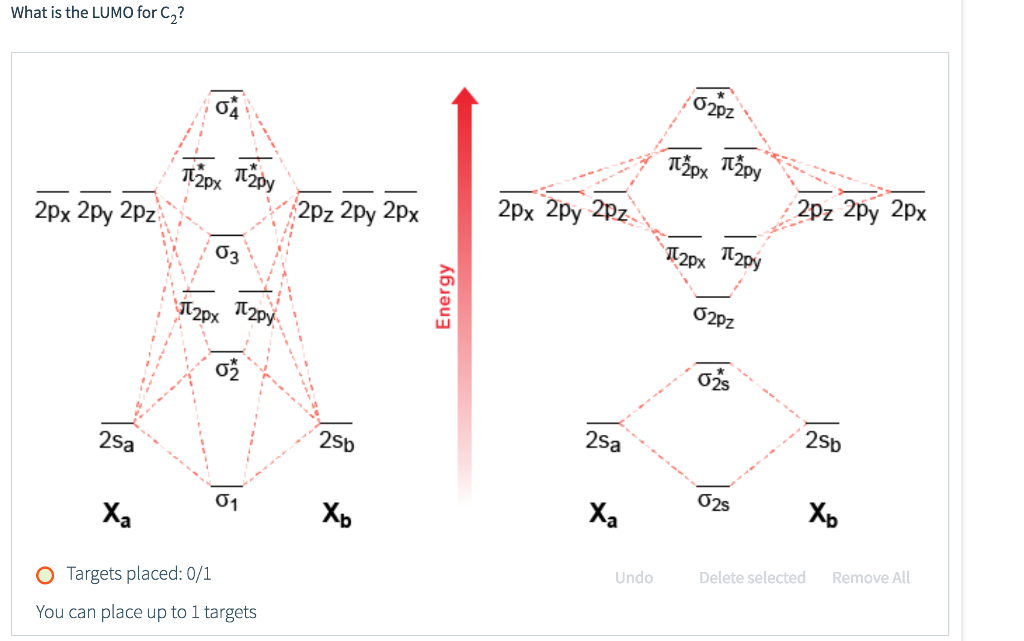
As you can see in the diagram, the two 2pπ orbitals, let’s say 2pπx and 2pπy , are the highest energy occupied molecular orbitals. The lowest energy unoccupied molecular orbital is 2pσ , so that is where the extra electron will be added.Dec 2, 2016
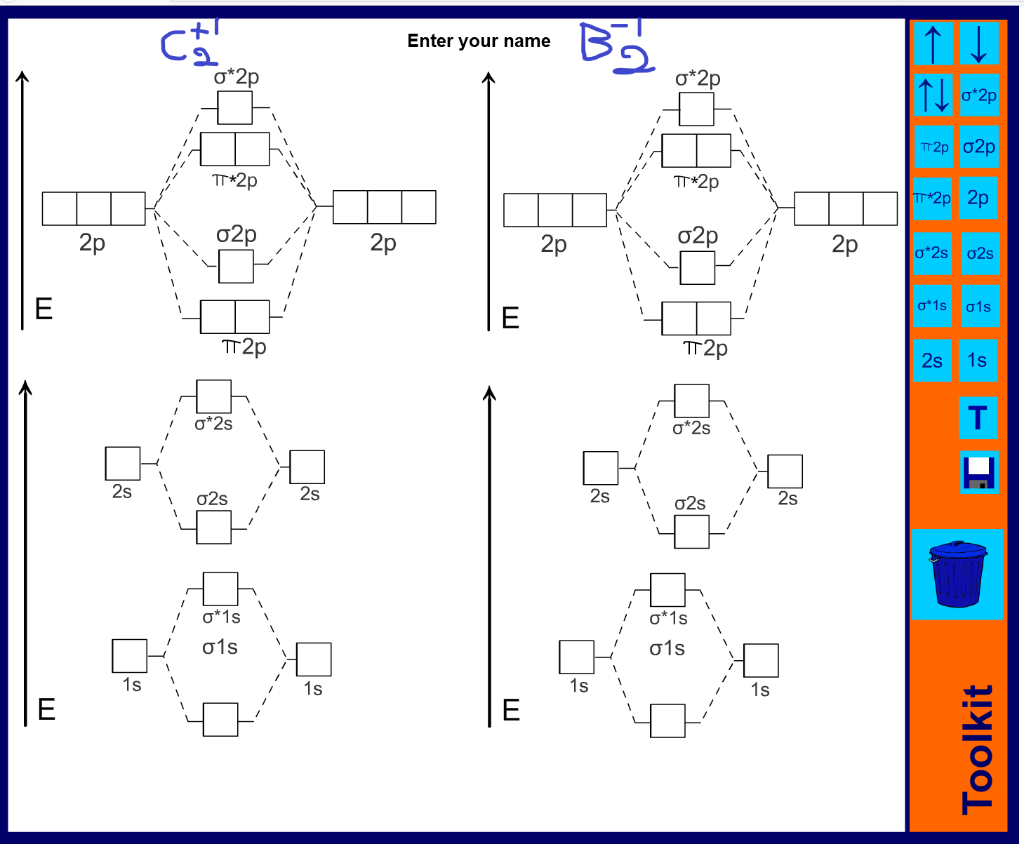
C2 molecular orbital diagram
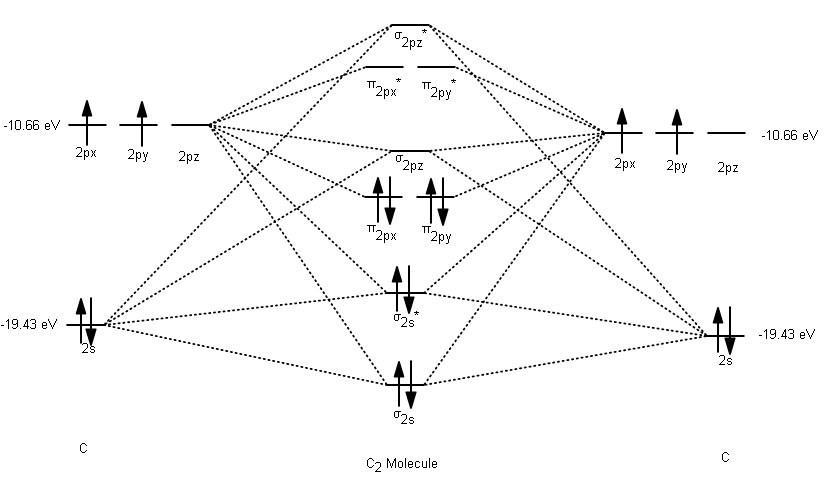
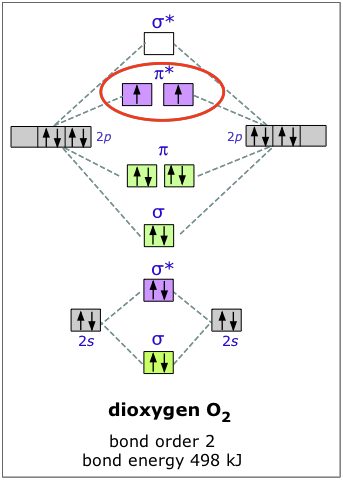
Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2.
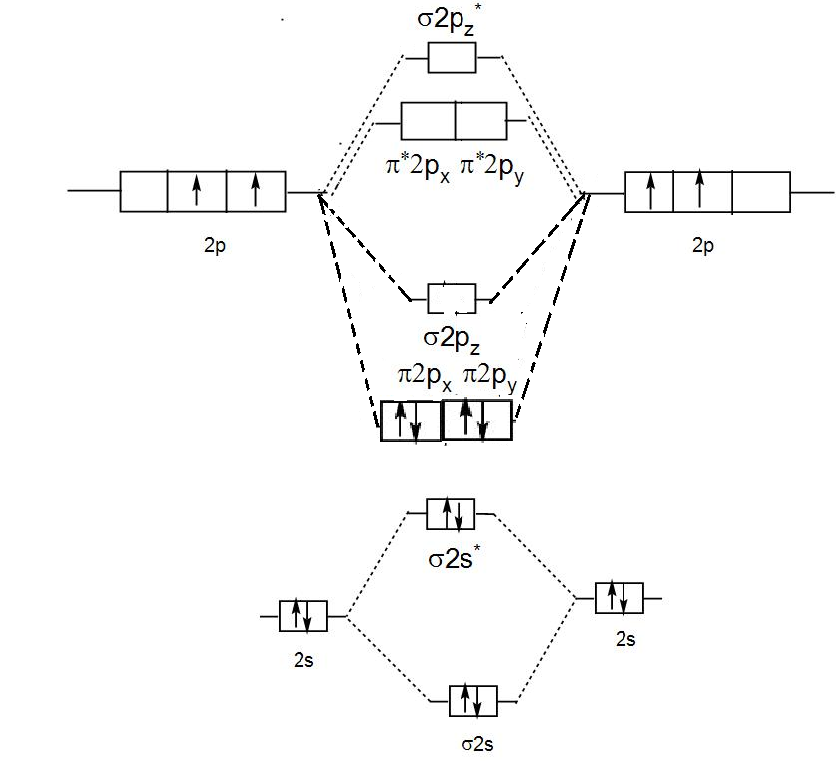
Molecular Orbital Theory shows that there are two sets of paired electrons in a degenerate pi bonding set of orbitals. This gives a bond order of 2, meaning ...6 answers · 8 votes: C2 exists, but only above 3,642 °C (6,588 °F) i.e. in vapor state
Molecular Orbital C2- Diagram, Bond Order, Magnetism. Postby Brooke Tobias 1B » Fri Nov 27, 2015 7:31 pm. A video explaining the molecular orbital diagram and notation of C2- along with the bond order and magnetism. You do not have the required permissions to view the files attached to this post. Top.
C2 molecular orbital diagram.
As you can see in the diagram, the two 2pπ orbitals, let's say 2pπx and 2pπy, are the highest energy occupied molecular orbitals. The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram
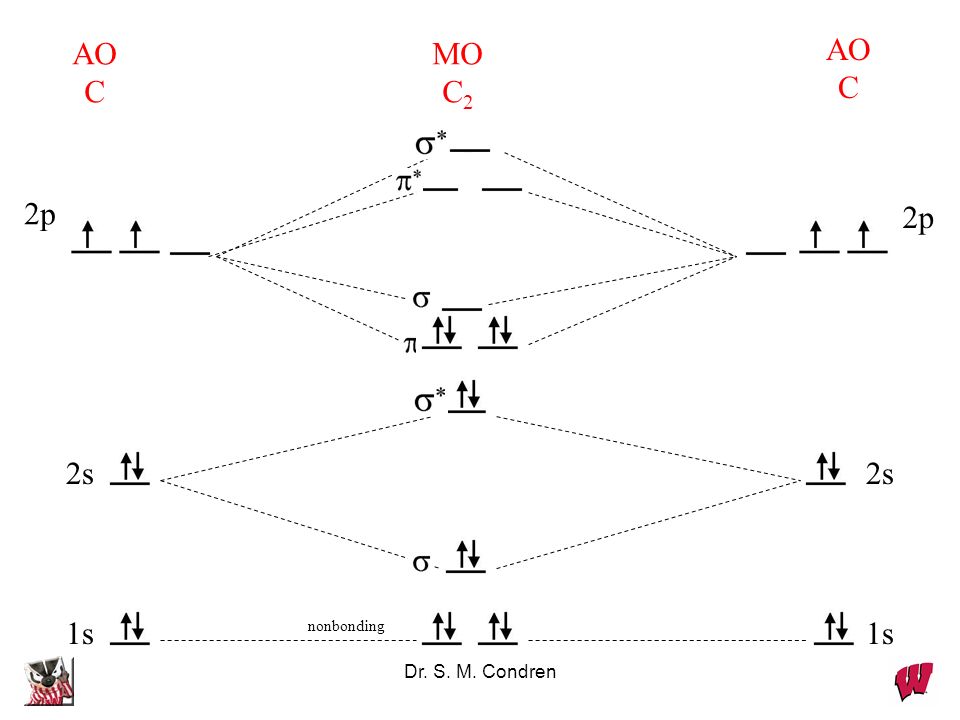
Molecular orbital theory shows that it has two sets of paired electrons in a degenerate bonding set of orbitals. This gives a bond order of two, which means that there should exist a double bond between the two carbons in a C 2 . As you know, a neutral carbon atom has a total of six electrons. This, of course, implies that a C 2 molecule has a ...
C2 molecular orbital diagram. In the mo approach each carbon atom has four valence orbitals namely a 2s and three 2p. Molecular orbital diagram for the molecule oxygen o2. The result is. Bonding order is 2 and it is diamagnetic. Bo 1 2 bonding e anti bonding e 1 2 8 4 2 lcao mo theory also predicts correctly that o2has two unpaired electrons.
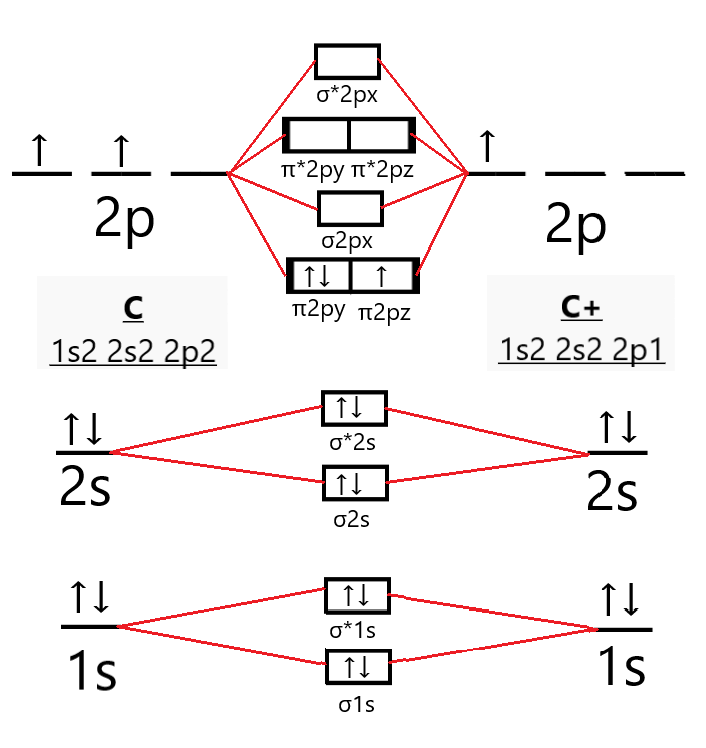
Chapter 1: Molecular Orbital Concepts A. Concepts of MO Theory. 1. Strong Covalent Bonds. Consider the pi bond of ethene in simple molecular orbital terms (The qualitative results would be the same for any pi or sigma bond.
Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Hydrogen | Fluorine | Nitrogen | Hydrogen Fluoride | Carbon Monoxide | Methane | Ammonia | Ethylene | Acetylene | Allene | Formaldehyde | Benzene
Antibonding orbital is type of molecular orbital (MO) that weakens the chemical bond between two atoms, whereas bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule.Thus the bond order of C2^- is 1/2 x (9-4) = 2.5 The bond order of C2− is paramagnetic because it has …
A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set. B) A bond order of 0 represents a stable chemical bond. C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in …
C2 molecular orbital diagram. A mo is defined as the combination of atomic orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined.
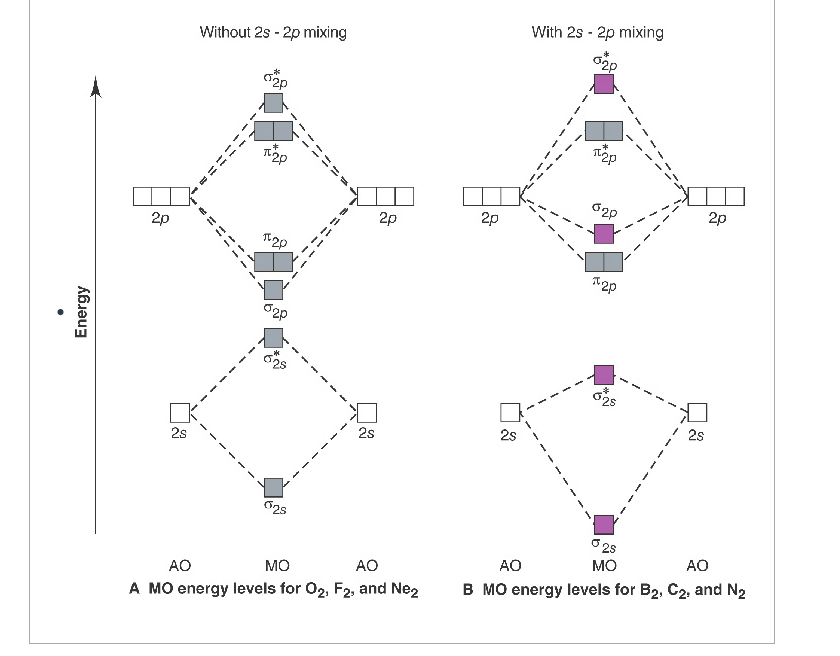
Place the following molecular orbitals in order of decreasing energy for species of B2, C2, and N2. start with the highest energy orbital. σ₂p* π₂p* σ₂p π₂p. Match each term with the appropriate electron arrangement paramagnetic diamagnetic. A molecular species with one or more unpaired electrons in an MO is _____ and will be attracted to a magnetic field, whereas a species with …
C2 molecular orbital diagram. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule. Get 11 help now from expert chemistry tutors. Fill from the bottom up with 8 electrons total. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms.
Molecular orbital theory shows that it has two sets of paired electrons in a degenerate $\pi $- bonding set of orbitals. This gives a bond order of 2, which ...
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
17.10.2018 · This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Energy level diagram for Molecular orbitals. …
(C2) Zero or even integer S A Odd integer A S Node When we move in molecular orbital, the sign of the orbital change (above the plane or consider all orbitals below the plane) is called as node. For a linear conjugated π-system the wave function ψ n will posses (n-1)nodes. If (n-1) is zero or even integer, ψ n will be said to be symmetric with respect to m and antisymmetric with …
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n (2px) 2 n (2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added.
26 Feb 2018 — write molecular orbital configuration of c2+ predict magnetic behaviour and calculate its bond order. ... The C2 molecule is diamagnetic because ...
C2 2- molecular orbital diagram The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2 : (1sσ)2 (1s* σ)2 (2sσ)2 (2s* σ)2 (2pπ)4. The electron configuration of the C− 2 ion will be.








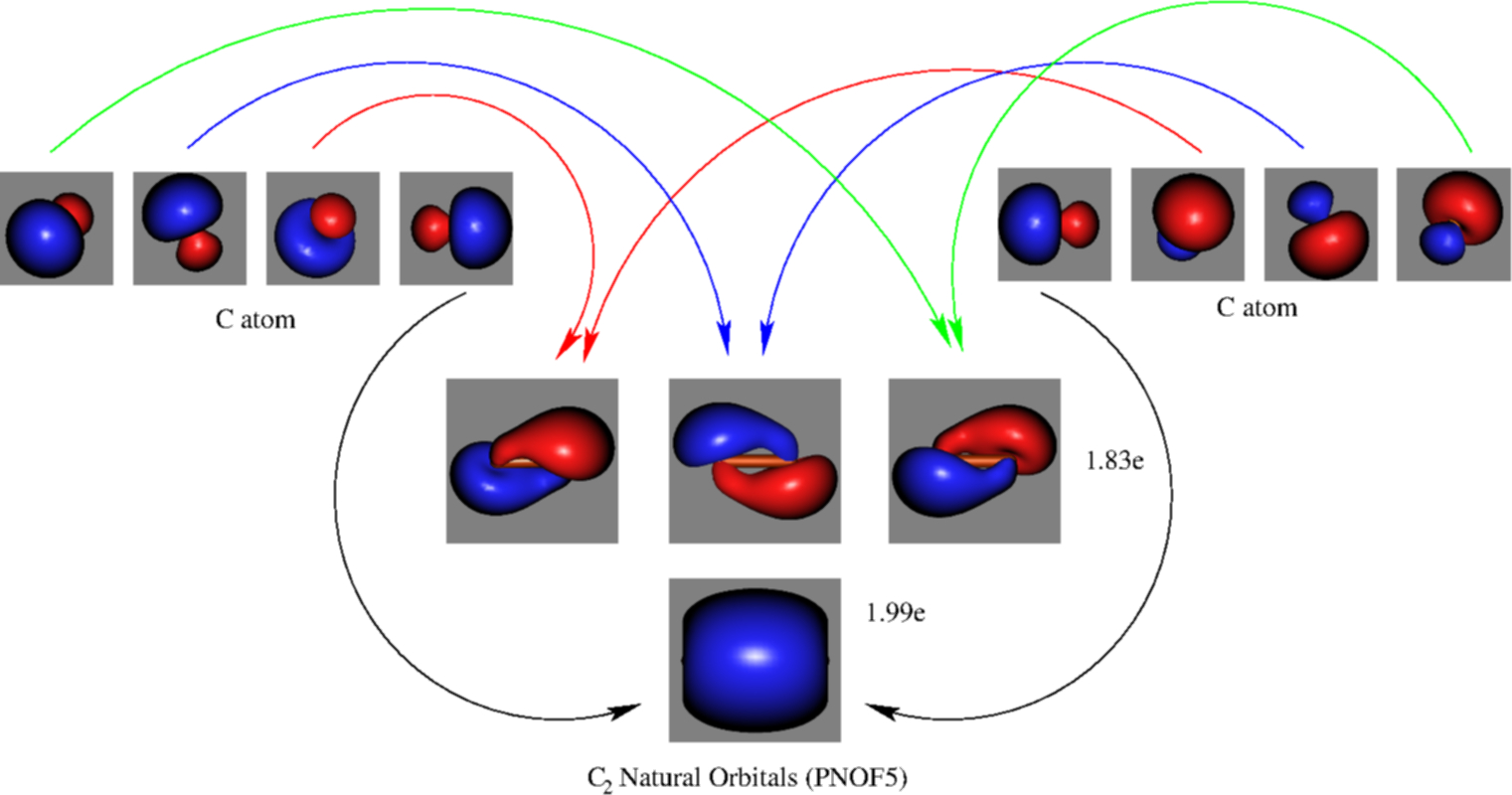
![Valence molecular orbitals of C2\documentclass[12pt]{minimal ...](https://www.researchgate.net/publication/337282871/figure/fig3/AS:958973926731776@1605648609153/Valence-molecular-orbitals-of-C2documentclass12ptminimal-usepackageamsmath.png)









0 Response to "38 c2 molecular orbital diagram"
Post a Comment