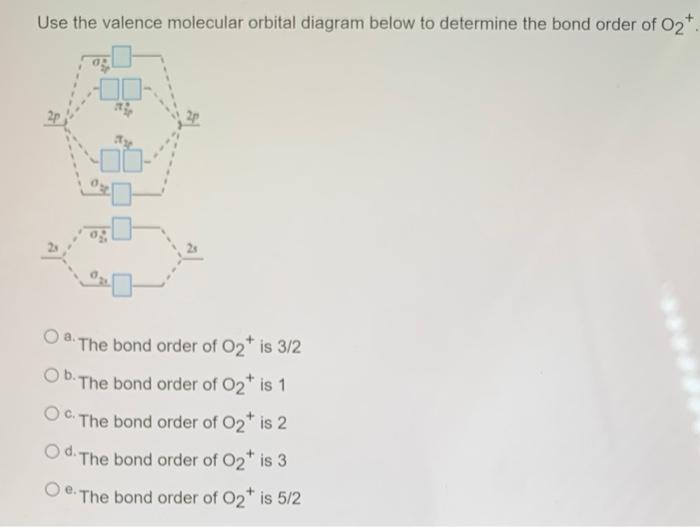
42 o2+ molecular orbital diagram
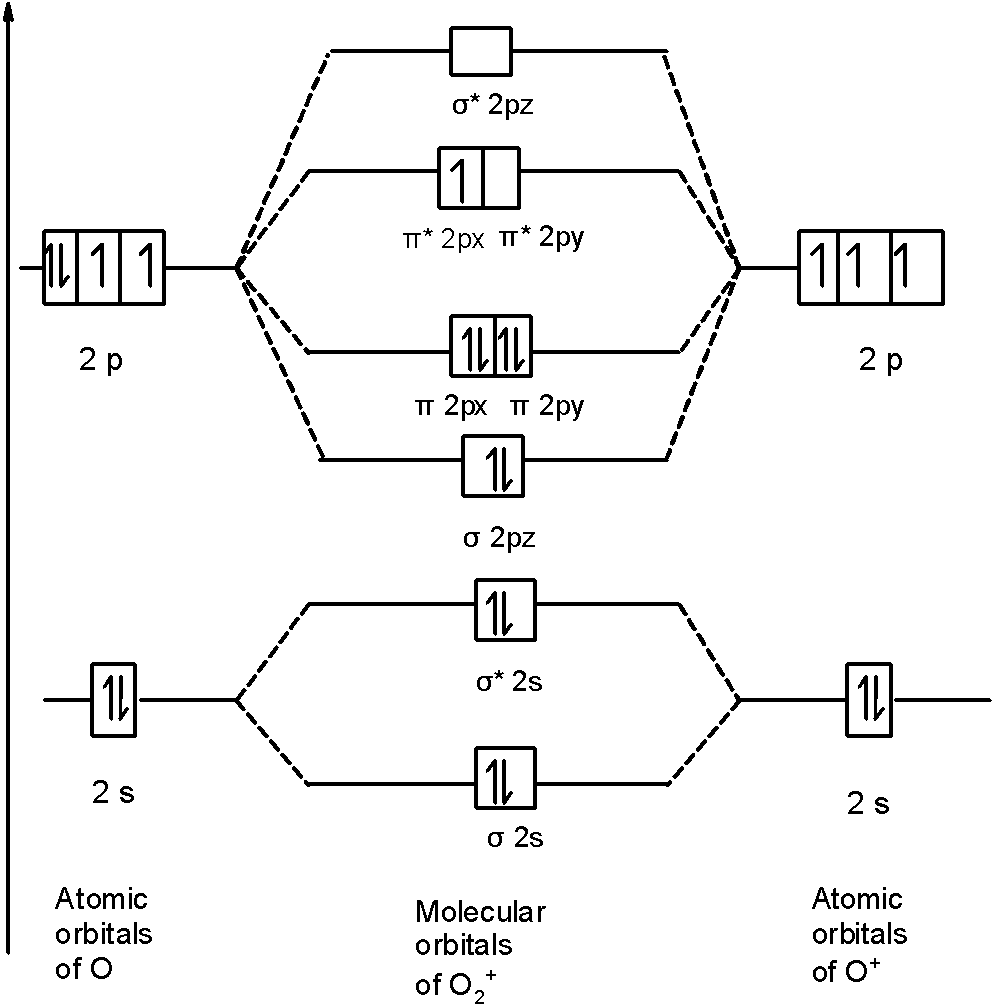
Mar 27, 2017 — From that diagram, you can then easily fill out what the O2- and O2+ MO diagrams should be—and that is in the second photo I included. The O2- MO diagram ...5 answers · 31 votes: Hello! I actually just covered this question in my gen chem class this week. I have attached ...What is the bond order of O2+? - Quora6 answersJan 31, 2018What's the MOT diagram of O2 +2 ion? - Quora3 answersAug 25, 2017What is the molecular orbital diagram of O2 and F2 ...6 answersMar 12, 2017What is the molecular orbital diagram for oxygen ...4 answersAug 20, 2015More results from www.quora.com Which is the correct molecular orbital diagram for O2+ ion? A. 01s 11 015* 1 1 025 1 1 023* TT2p 1 1 0 2p TT2p* 11,1 B. 01s 1 01s* 1 1 025 1 1 023* 1 1 1T2p 1 1 O2p 11 TT2p% 11,1 C. 01s 1101s* 1 1 025 1 1 023* 1 TT2p T 1, 1 1 0 2p ſ TT2p 11 D. 015 1 1 015* 1 1 025 1 1 028" 1 TT2p 11, 11 o2p 11 TT2p* 1 25.
The bond order varies from one molecule to another. Oxygen is a diatomic molecule. Let us first know what is meant by bond order. Bond order. The bond order may be defined as half the difference between the number of electrons in bonding molecular orbitals (Nonbonding) and the number of electrons in the antibonding molecular orbital. Formula

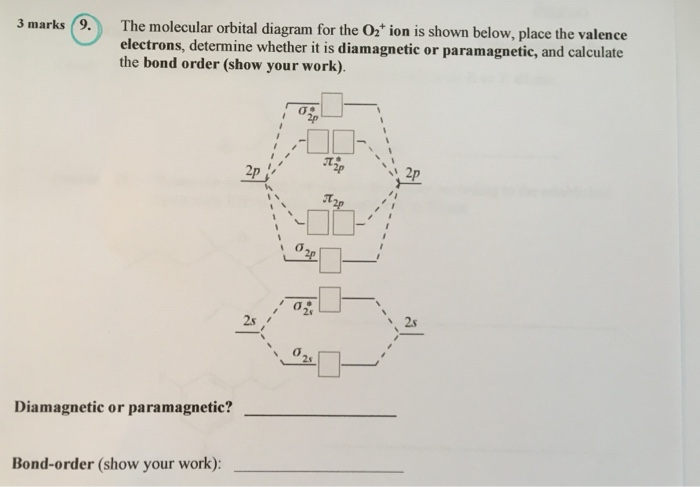
O2+ molecular orbital diagram
You'll need the molecular orbital (MO) diagram of "O"_2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get "O"_2. Two 2s orbitals combine to give a sigma_(2s) bonding and sigma_(2s)^"*" antibonding MO. 1 answerAs it can be seen from the given structures that in the molecular orbital diagram for O2+ ion, the highest occupied orbital is π∗ MO orbital. Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
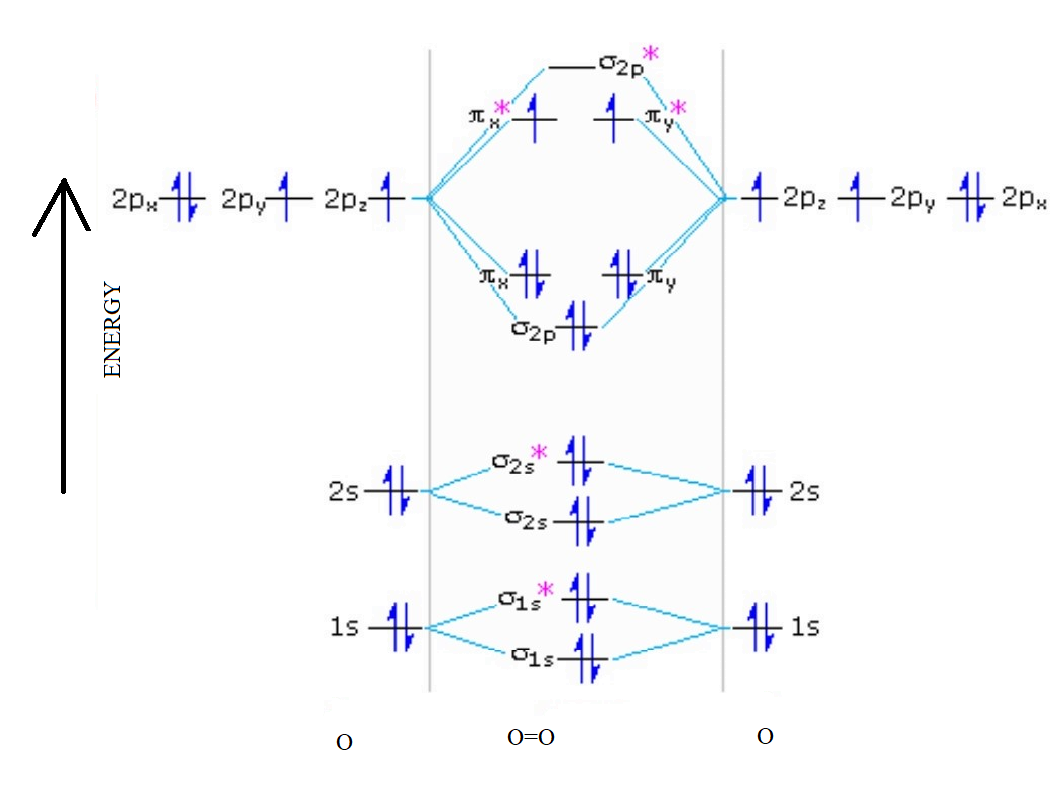
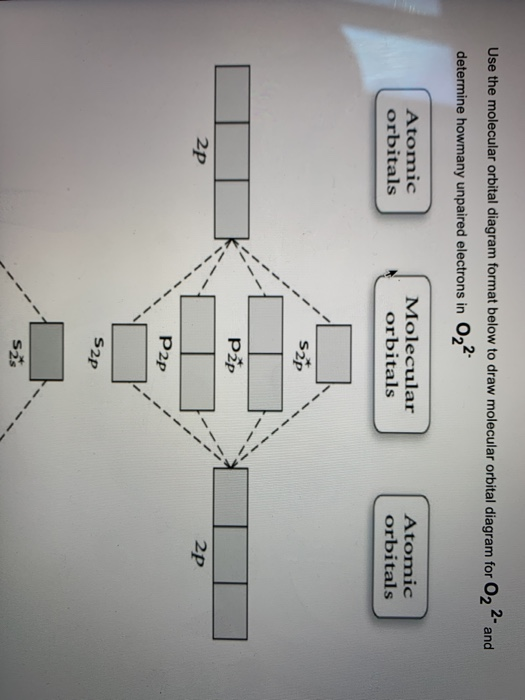
O2+ molecular orbital diagram. We know that Oxygen has atomic number = 8. Thus, the electronic configuration for an atom of oxygen in the ground state can be given as - $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the othe... Answer to: Construct Molecular Orbital Diagram and determine unpaired electrons in O2- , O2+ , BN , NO- By signing up, you'll get thousands of...1 answer · Top answer: The MO diagram of O−2O2− is O−2O2− contains one unpaired electron The MO diagram of O+2O2+ is {eq}O_2^+ {/... Draw similar diagrams for other orbitals in the print out.The first photo is straight from a edition Pearson general chemistry textbook, and it shows you what the molecular orbital (MO) diagram for O2 is. From that diagram, you can then easily fill out what the O2- and O2+ MO diagrams should be—and that is in the second photo I included.
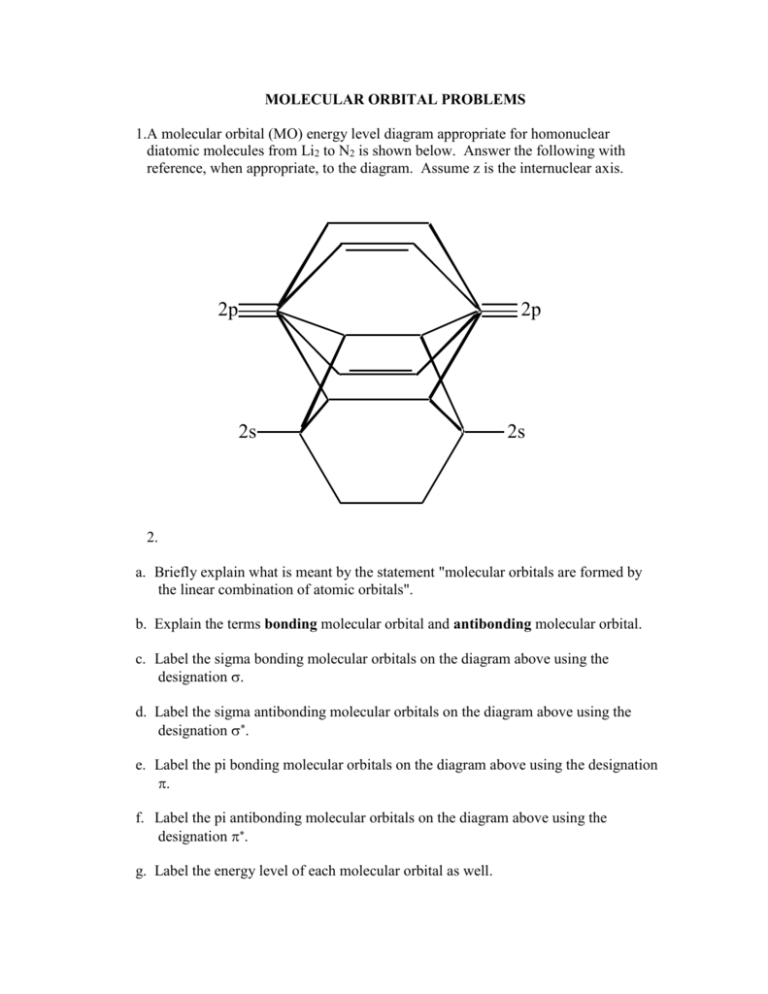
The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Learn about the molecular orbital diagram for o2 using these free and printable molecular orbital diagram as your reference in understanding the mo of oxygen. The other four p-atomic orbitals (two from each oxygen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two antibonding molecular orbitals i.e. π*2py and π*2pz. The electron filling in these molecular orbitals follow aufbau, pauli exclusion principle and hund's rule. Formation of Molecular Orbitals. An atomic orbital is an electron wave; the waves of the two atomic orbitals may be in phase or out of phase. Suppose Ψ A and Ψ B represent the amplitude of the electron wave of the atomic orbitals of the two atoms A and B. Case 1: When the two waves are in phase so that they add up and amplitude of the wave is ... The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
May 19, 2014 — Because According to molecular orbital theory O2+ has 15 electrons &it has one electron in antibonding orbital. molecular orbital diagram of ... Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. 5.7 a. The energy level diagram for NO is on the right. The odd electron is in a π2p* orbital. b ...29 pages Vol. 8, No. 95 · Magazine3 LU Atomic orbitals Molecular orbitals Atomic orbitals Molecular orbital ... of one electron from O2 molecule O2 - e~ > O2+ From MO diagram of O2 molecule, ...

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are
O2 molecular orbital diagram oxygen has a similar setup to h 2 but now we consider 2s and 2p orbitals. This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals. Beyard 3.
Figure 9.39: The molecular orbital energy-level diagrams, bond orders, bond energies, and bond lengths for the diatomic molecules B 2 through F 2 .Note that for O 2 and F 2 the σ2p orbital is lower in energy than the π 2p orbitals.
On a very general basis, electrons are not assigned to individual bonds between atoms, but they move under the influence of the nuclei in the whole molecule. Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ .
molecular orbital diagram of O2-Electronic configuration of O2-itemderby itemderby Explanation: In a molecule, there are total 16 electrons. The molecular orbital configuration of molecule is as follows. The formula for bond order is as follows. Bond order = There are 10 bonding and 6 non-bonding electrons in the orbitals according to the ...
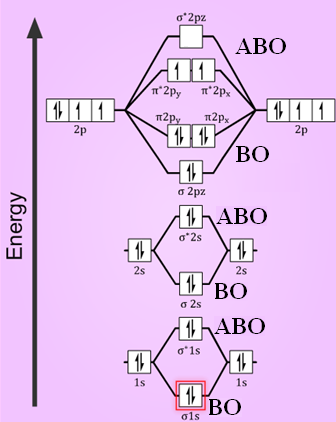
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. It also provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. More molecular orbital diagrams for 02 are provided below.
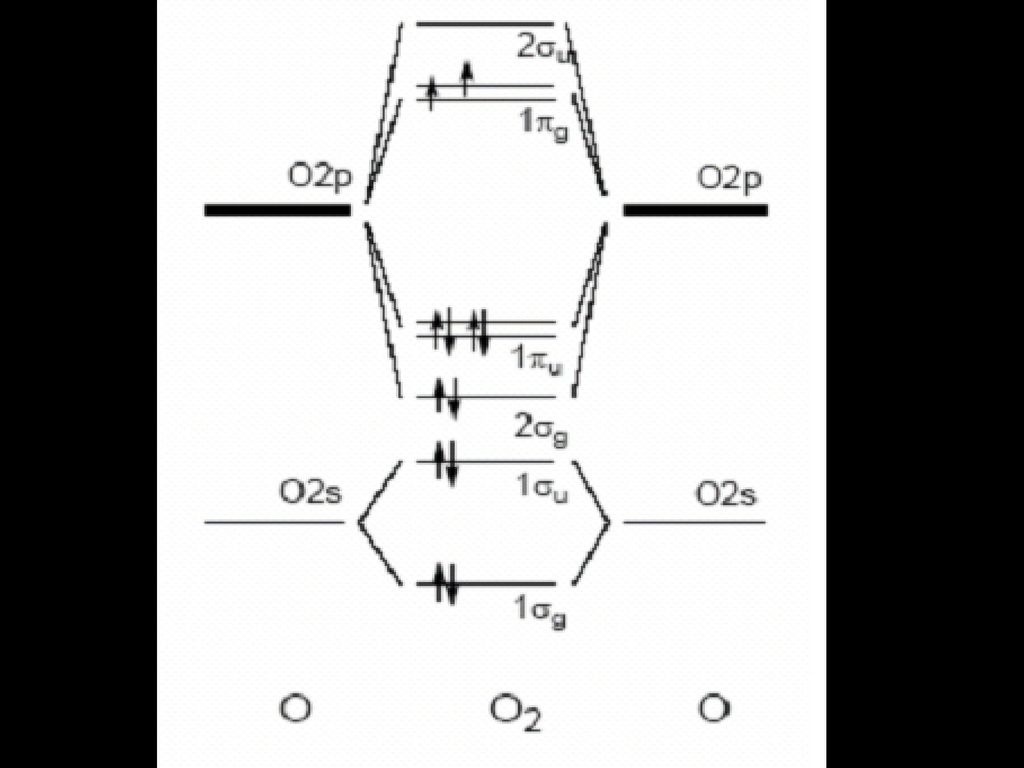
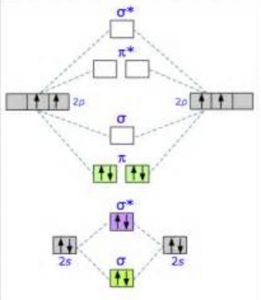
To obtain the molecular orbital energy-level diagram for (ce {O2}), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure (PageIndex {1}). We again fill the orbitals according to Hund's rules and the Pauli principle, beginning with the orbital that is lowest in energy.
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
sorry about that not being a molecular orbital diagram i saw orbital and immediately thought electron configuration to save confusion, could. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen.
Complete this valence molecular-orbital diagram for | Chegg.com. Science. Chemistry. Chemistry questions and answers. Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. Question: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as ...
The only orbitals that are important in our discussion of molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.

Molecular Orbital Theory Mot Designation Of Mo Formation Of S S Mo Electronic Configuration Of Species Behaviour Of Molecules Bond Order Bond Energy Bond Length Magnetic Behaviour
The double bond in C 2 consist of both Pi bonds because the four electrons are present in the two pi molecular orbitals. 10) N 2. ... Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e-. Reply. Mrs Shilpi Nagpal says. September 26, 2018 at 11:06 am.
Hint: First draw a molecular orbital diagram (MOT) where the atomic orbitals combine to form molecular orbitals. The total electrons associated with the ...
Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ...

Consider The Molecular Orbital Diagram For The Ion O 2 2 Predict The Bond Order A 3 0 B 2 5 C 1 0 D 2 0 E 1 5 Consider The Following Statements Will The Ion Be Paramagnetic Or Study Com
The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond.
Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
1 answerAs it can be seen from the given structures that in the molecular orbital diagram for O2+ ion, the highest occupied orbital is π∗ MO orbital.
You'll need the molecular orbital (MO) diagram of "O"_2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get "O"_2. Two 2s orbitals combine to give a sigma_(2s) bonding and sigma_(2s)^"*" antibonding MO.

Mo Diagram Of O2 O2 O2 Their Bond Order And Magnetic Char Chemistry Chemical Bonding And Molecular Structure 2562266 Meritnation Com
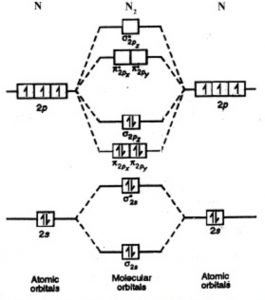
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

Calculate The Number Of Unpaired Electrons In O2 And O2 2 Ions By Drawing Molecular Orbital Brainly In

Can You Please Explain Me Molecular Orbitals Concept And The Way To Draw Their Structures Chemistry Chemical Bonding And Molecular Structure 11682223 Meritnation Com

















0 Response to "42 o2+ molecular orbital diagram"
Post a Comment