42 label the energy diagram for a two step reaction
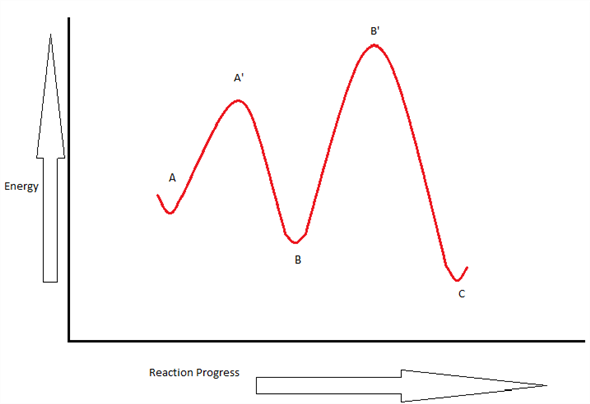
The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 .Each elementary step has its own activated complex, labeled AC 1 and AC 2 .Note that the overall enthalpy change of the reaction is unaffected by the individual steps, since it depends only on the initial and final states. Question. Draw an energy diagram for a two-step reaction, A → B → C, where the relative energy of these compounds is C <; A < B, and the conversion of B → C is rate-determining. check_circle.
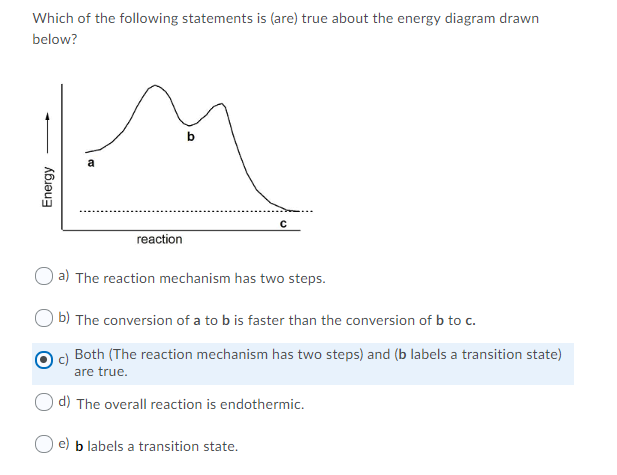
A Two-Step Reaction Mechanism. The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Step 1 has the higher transition energy state, thus it ...
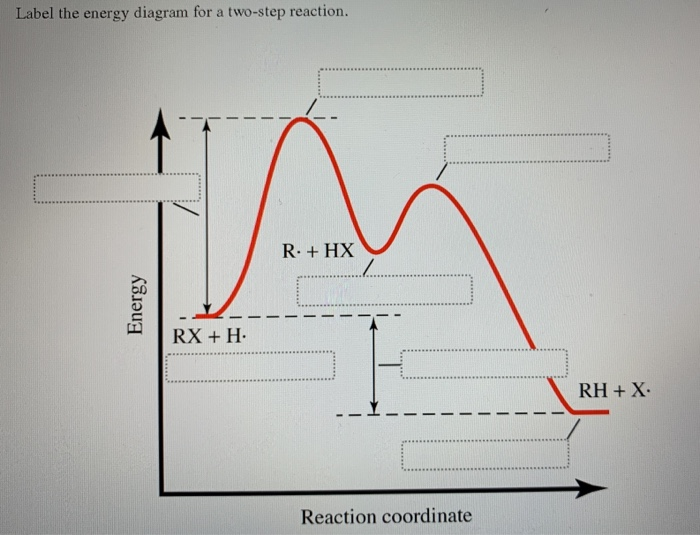
Label the energy diagram for a two step reaction
An enthalpy diagram allows us to easily see details of a chemical reaction. By knowing how to draw and label an enthalpy diagram we can see what the starting energy level is, how much energy is ... A Two-Step Reaction Mechanism We draw an energy diagram for each step, and then combine them in an energy diagram for the overall two step mechanism. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. Label this diagram energy reaction progress. Label the graph with each of the following. Label the energy diagram for a two step reaction. 2 i dont understand. It also shows the effect of a catalyst on the forward and reverse activation energy. Show transcribed image text.
Label the energy diagram for a two step reaction. Start studying Labeling an Energy Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Label the energy diagram for a two step reaction. I need the actual answer for the rate constant number and the units please. Each step has its own delta h and activation energy. In the s n 1 reaction the carbocation species is a reaction intermediate. The transition states are located at energy maxima. Label the energy diagram for a two step ... Label the energy diagram for a two-step reaction. Q. A reaction coordinate diagram is shown below for the reaction of A to form E. Answer the following questions.i) Identify the transition state (s)?ii) W... Q. Which reaction coordinate diagram represents a reaction in which the activation energy, Ea, is 50 kj.mol-1 and the ΔHrxn is -15 kj. mol-1? Label the energy diagram for a two step reaction. For exothermic reactions the reactants are drawn above the products because their energy is greater. 2 i dont understand. The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram or sometimes called a reaction progress curve.
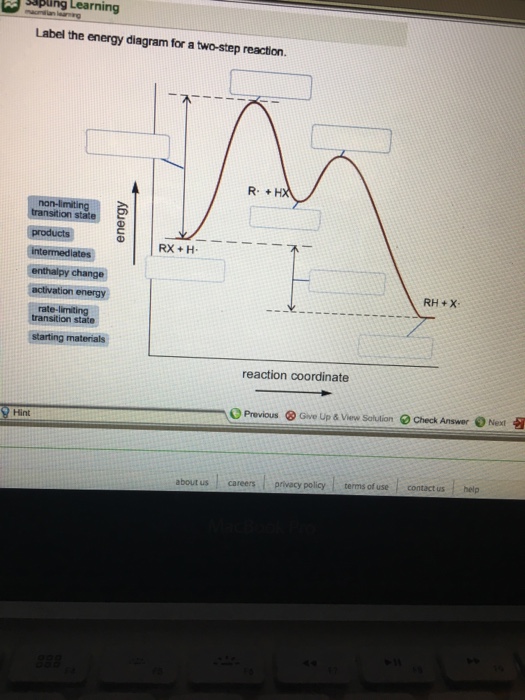
See the answer See the answer done loading. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (42 ratings) Transcribed image text: Label the energy diagram for a two-step reaction. You will place all the labels in part a. Show more 1 label the energy diagram shown below for a two step reaction. Label the energy diagram and answer the question that follows. Each label can be used only once. Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram for a two-step reaction. Subject: Chemistry Price: 3.85 Bought 3 Share With. Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate Label The Energy Diagram For A Two Step Reaction Clutch Prep The solid line in the energy diagram represents changes in energy as the reactant is converted to product under standard conditions. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. The reaction of no with o2 provides an alternative reaction ...
here for the reaction. Coordinate diagram. Put two step verification in which the first step, Andrea Janek, the second step is extra, Janek. And the order reaction is and a sonic labels are re intense. Products, intermediates and chronic transition is start stairs. So this is the reaction co ordination diagram in which this is for free energy, the bikes and X axis for the the reaction coordinates. a. Draw an energy diagram for a two-step reaction, A B C, where the relative energy of these compounds is A<C<B, and the conversion of A B is rate determining. b. Label H° and Ea for each step and label H° overall. c. Label each transition state. d. Which point on the graph corresponds to a reactive intermediate? e. 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. step in a multistep reaction. EXAMPLE 5.1 Draw an energy diagram for a two-step exothermic reaction in which the second step is rate determining. STRATEGY A two-step reaction involves the formation of an intermedi-ate. In order for the reaction to be exothermic, the products must be lower in energy than the reactants. In order for the
We have solutions for your book! Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall Δ G °, transition states, and intermediate. Is Δ G ° positive or negative? Gibb’s free-energy is the energy difference between the reactants and the products.
Draw an energy diagram for a two-step reaction, A $\rightarrow$ B $\rightarrow$ C, where the relative energy of these compounds is C <; A < B, and the conversion of B $\rightarrow$ C is rate-determining.
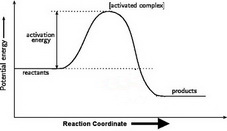
1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4.
57CP. Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, ΔH°, and E a. a. A concerted, exothermic reaction with a low energy of activation. b. A one-step endothermic reaction with a high energy of activation. c. A two-step reaction.
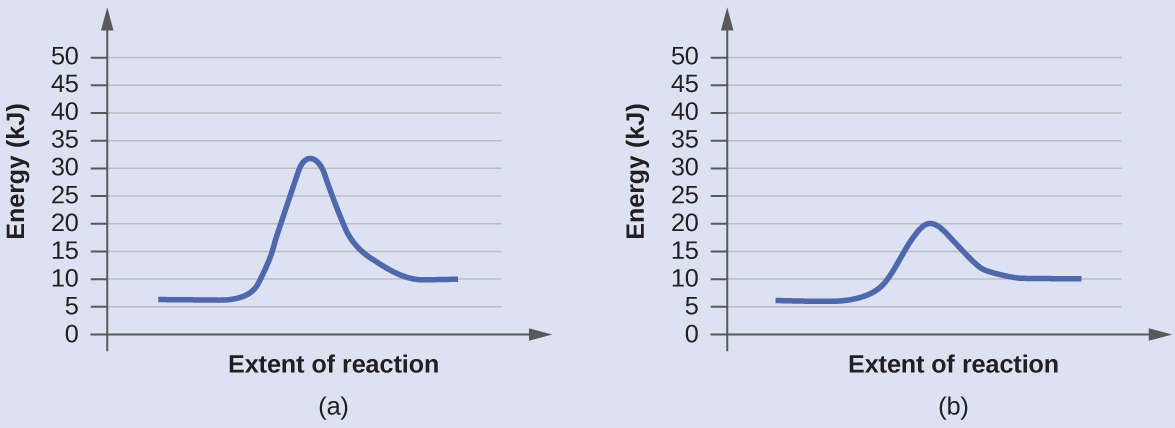
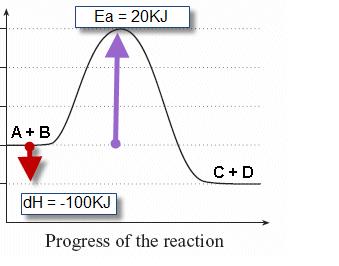
Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
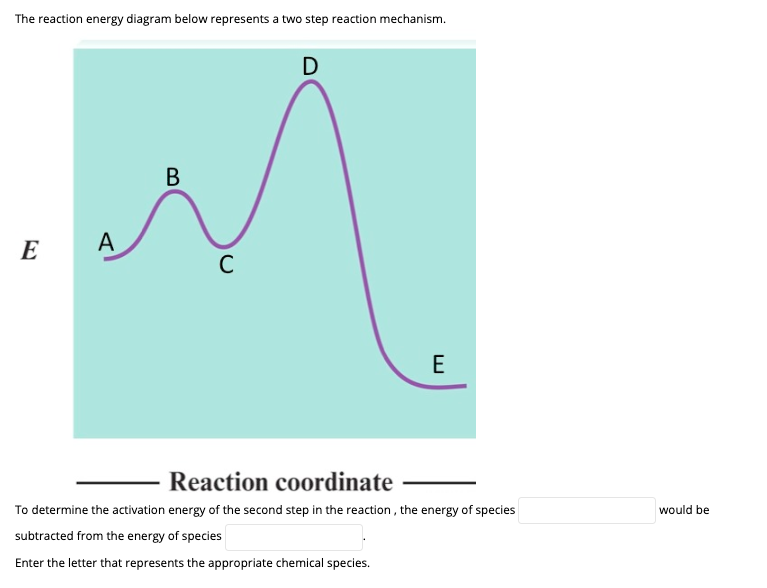
We're being asked to label the given energy diagram.. Recall that an energy diagram is usually read from left to right.The components of a two-step energy diagram are: • Reactants: are placed on the left/beginning of the energy diagram • Products: are placed on the right/end of the energy diagram • Non-limiting transition state: is the transition state with the lowest energy in the ...
Feb 03, 2020 · Each elementary step has its own activated complex labeled ac 1 and ac 2. Show more 1 label the energy diagram shown below for a two step reaction. Reaction Coordinate Wikipedia The activation energy for each step is labeled e a1 and e a2. Label the energy diagram for a two step reaction. It also shows the effect of a catalyst on the forward and reverse activation energy.
Label the following multi-step reaction energy diagram. viridianmosquito683 Draw an energy diagram for a two-step reaction where the first step is exothermic and the reaction overall is endothermic.
1. Identify the major product for the reaction below and provide a detailed reaction mechanism to account for its formation: Label the rate determining step (RDS)* HCI- Major Product Using the reaction above, fill in the reaction energy diagram and clearly indicate and CLEARLY label each of the following: 2.
Solved Draw An Energy Diagram For A Two Step Exergonic Reaction Whose Second Step Is Faster Than Its First Step
65AP. Draw a reaction energy diagram for a two-step reaction that has an endothermic first step and an exothermic second step. Label the reactants, transition states, reaction intermediate, activation energies, and enthalpy differences. Step-by-step solution. 100% (4 ratings) for this solution. Chapter 3, Problem 18P is solved. View this answer.

Solved Question 4 Of 11 12 Points Draw An Energy Diagram For Two Step Reaction That Meets The Criteria Listed Below Start By Drawing Your Axes Similar To The Image Below Energy On The
The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ...
This video looks at the features of a reaction energy diagram and how you can use it to interpret and understand the mechanism of a reaction.
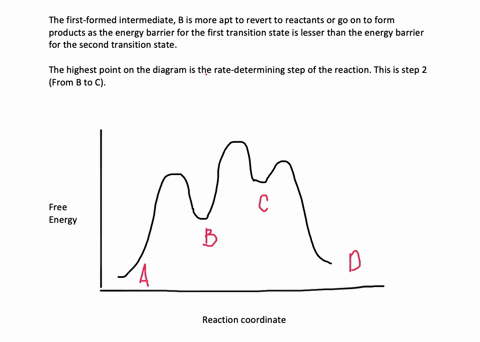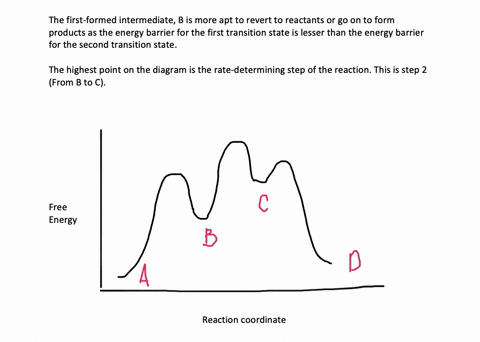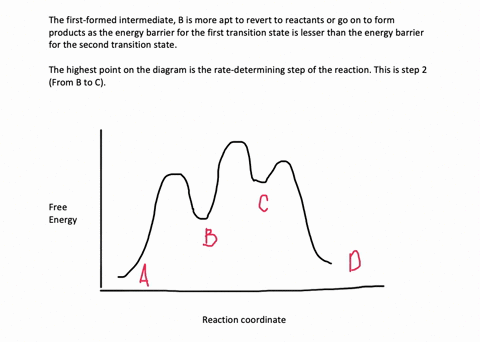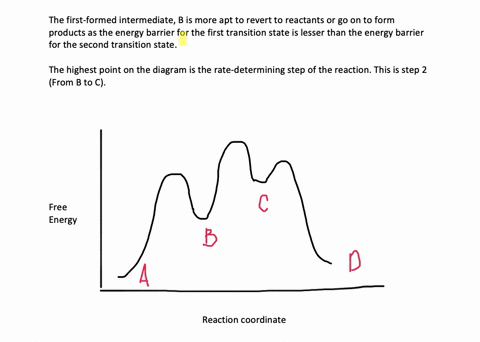
is the complex created in the first reaction, while is the activated complex created in the second reaction. Thus, for this two-step process, there are two activated complexes. Example: Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step

Choose The Correct Reaction Coordinate Diagram For A Two Step Reaction In Which The First Step Is Homeworklib
5. Question 5 – The Energy Diagram of SN2 reaction: Draw an energy diagram for the following S N 2 reaction. Label the axes, the Ea, the ΔH° and the transition state of the reaction.Assume the reaction is exothermic and ΔH° = -75 kJ/mol and Ea = 50 kJ/mol. Draw the structure of reactants and products on the diagram.You can put the reactants at any energy level and then draw the rest as ...
Label the energy diagram for a two step reaction. For 1 id go in this order left to right. Expert answer 100 2 ratings. And from now on this is the very first picture. Label the energy diagram for a two step reaction. Draw a reaction energy diagram for a two step endothermic reaction with a rate limiting second step.
Watch Complete videos @ www.LearningChemistryOnline.com Organic Chemistry 1
The rate constant for any step is inversely related to its activation energy; the higher the activation energy, the smaller the rate constant Self-test question #1. Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step.
Label this diagram energy reaction progress. Label the graph with each of the following. Label the energy diagram for a two step reaction. 2 i dont understand. It also shows the effect of a catalyst on the forward and reverse activation energy. Show transcribed image text.
A Two-Step Reaction Mechanism We draw an energy diagram for each step, and then combine them in an energy diagram for the overall two step mechanism. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram. Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram.
An enthalpy diagram allows us to easily see details of a chemical reaction. By knowing how to draw and label an enthalpy diagram we can see what the starting energy level is, how much energy is ...
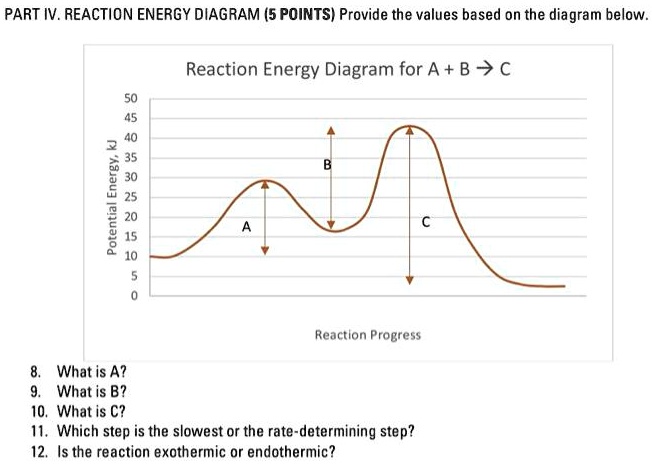
Solved Part Iv Reaction Energy Diagram 5 Points Provide The Values Based On The Diagram Below Reaction Energy Diagram For A B V A 1 1 Reaction Progress What Is A What
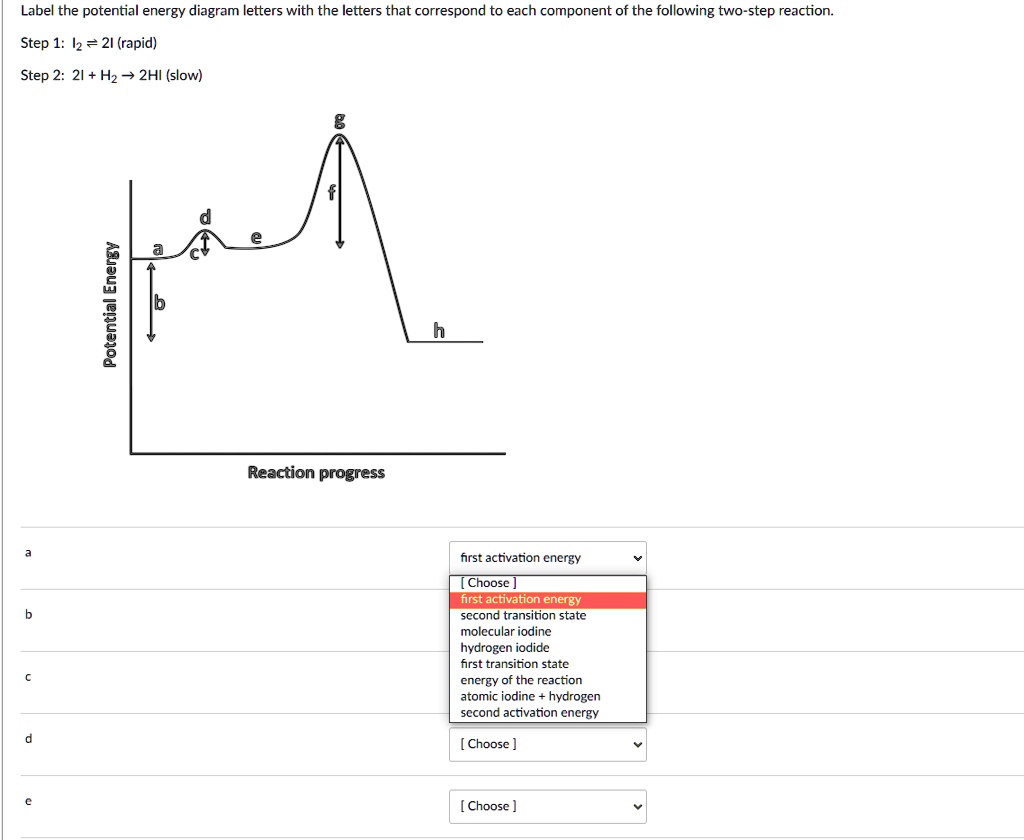
Solved Label The Potential Energy Diagram Letters With The Letters That Correspond To Each Component Of The Following Two Step Reaction Step 1 Iz 2i Rapid Step 2 21 Hz 2hi Slow
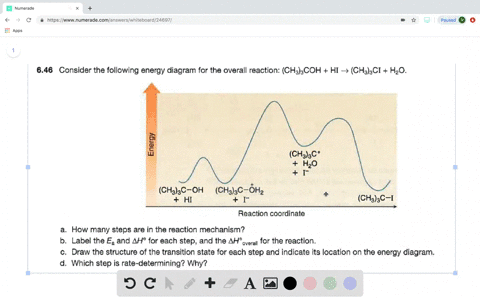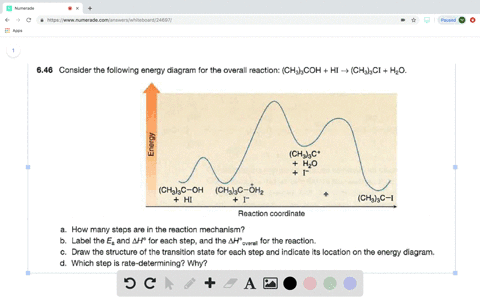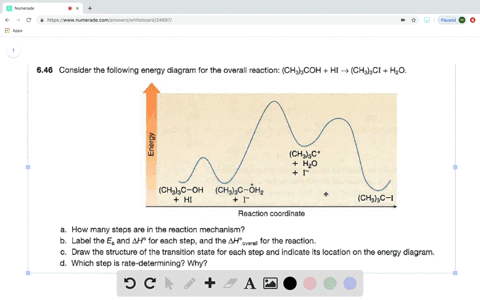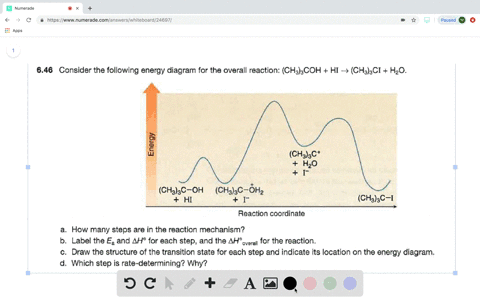
Solved Consider The Following Two Step Reaction A How Many Bonds Are Broken And Formed In Step 1 Would You Predict Delta H Circ Of Step 1 To Be Positive Or Negative B How Many Bonds
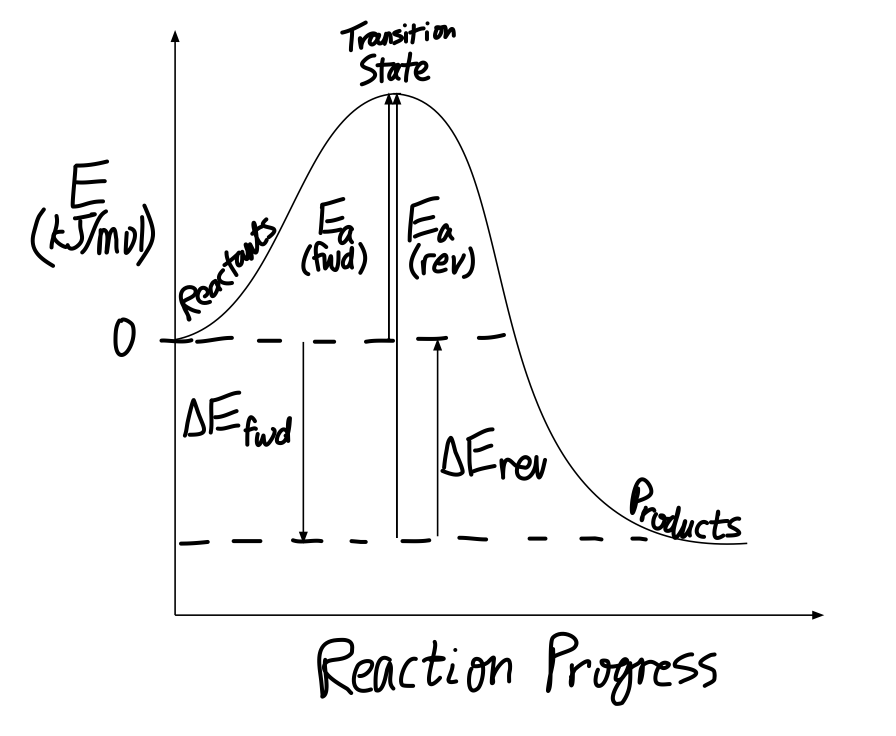
How Would You Draw And Label Energy Diagrams That Depict The Following Reactions And Determine All Remaining Values Place The Reactants At Energy Level Zero Socratic
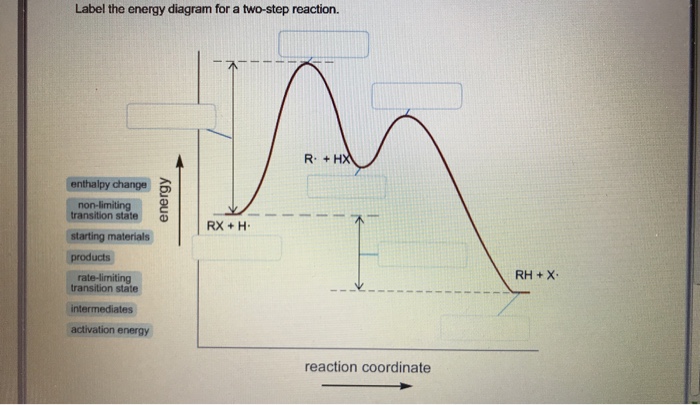

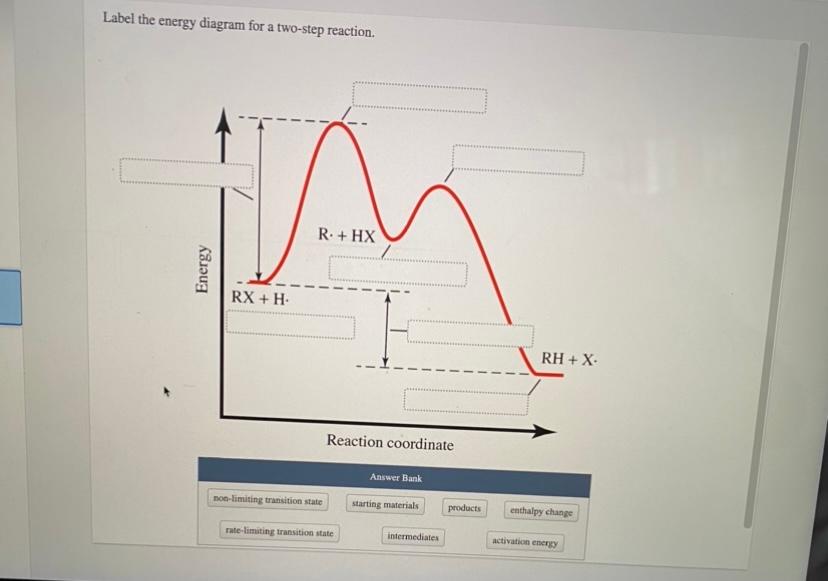

















0 Response to "42 label the energy diagram for a two step reaction"
Post a Comment