41 mo diagram for h2
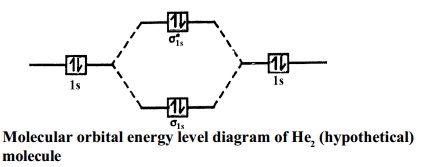
Molecular Orbital Diagram He2. This molecular orbital treatment can explain why H2 exists but He2 does not. Draw a complete MO diagram for all the bonds in ethene. He2 is not possible. He MO Diagram. Eg: He + H; same mixing as above. Three electrons, two in sigma, one in sigma*. One more electron in. Answer to Construct the molecular orbital ... Answer: According to the molecular orbital theory, a molecular is viable of its bond order is more than or equal to one. The bond order is defined as number of bond between to two atoms of that molecule. It is calculated as the difference of electrons in bonding molecules and anti-bonding molecul...
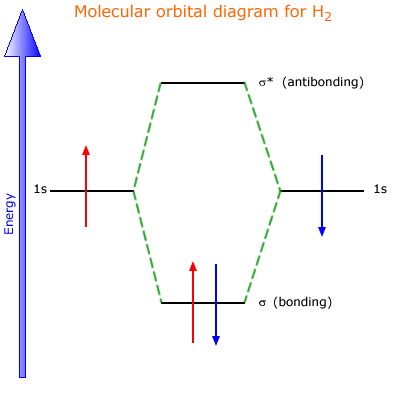
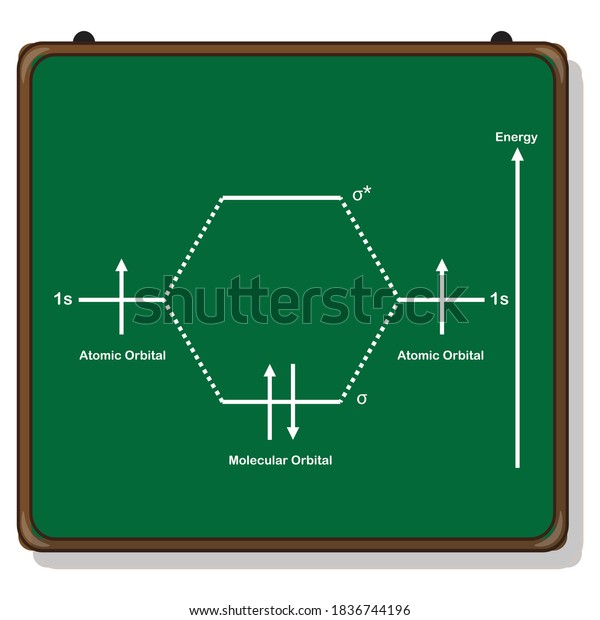
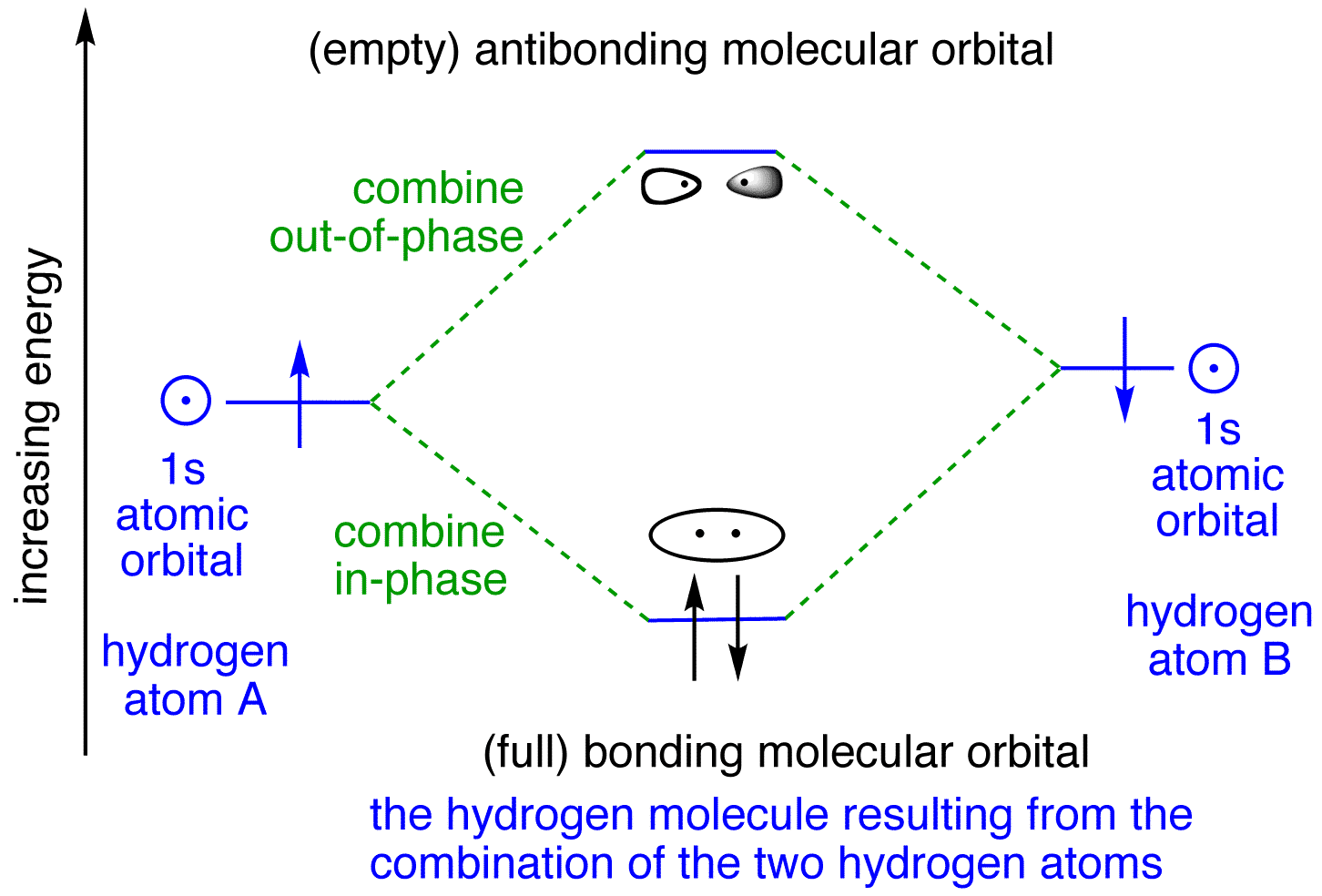
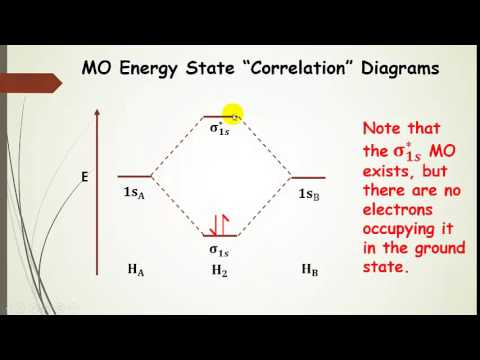
Chemistry questions and answers. The below Mo diagram is for H2. A hydrogen atom contains one valence electron, therefore the is atomic orbital contains a single electron. This is true for each hydrogen atom. The middle of the MO diagram shows the molecular orbitals that have been filled with the valence electrons from the original atoms.

Mo diagram for h2
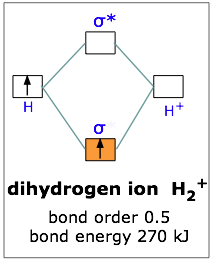
The energy curves for ψ + and ψ-reveal the following properties of the ion H 2 +. The curve for ψ + refers to the ground state of the molecule where a minimum energy is found for a nuclear distance of approximately 2a o (i.e. 100pm). Thus, H 2 + should exist as a stable molecule. The calculated bonding energy is 1.77 eV. This is a quite satisfying result; from experiments we get 2.77 eV. Draw mo energy diagrams for the molecular ions h2 and h2. Construct the molecular orbital diagram for h2. When the 1s wave functions of the two ceh atoms are linearly combined we get a sigma s bonding orbital denoted as s 1s in the diagram herethis approach is called linear combination of atomic orbitals lcao. Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
Mo diagram for h2. This molecular orbital treatment can explain why H2 exists but He2 does not. Draw a complete MO diagram for all the bonds in ethene.© Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window. Show transcribed image text Construct the molecular orbital diagram for He2 and then identify the bond order. Answer and Explanation: 1. This question deals with the molecular orbital (MO) diagram of the simple diatomic hydrogen molecule. VSEPR theory describes this as two hydrogen atoms forming a single ... Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals. Molecular Orbital (MO) Theory of the H2 molecule: ... Two electrons in Molecular Orbital ψ_+ 2) One electron in MO ψ_+ and one electron in MO ψ_‐ 3) Two electrons in MO ψ_‐. ... Qualitative MO theory orbital diagram for homonuclear diatomics composed of 1st or 2nd row elements:
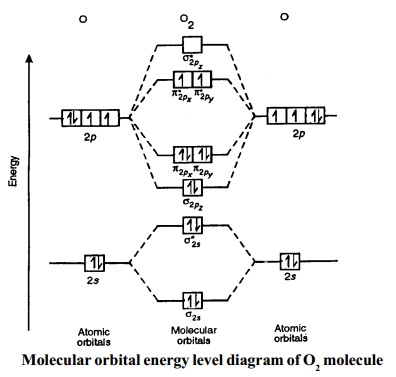
1) H2- 2) H2+ 3) H2 4) He2+. A species is said to be diamagnetic when it has all the paired electrons. Similarly if the species contain unpaired electron it is said to be paramagnetic. To know the magnetic character of molecules we can use MO diagram. When we draw MO diagram for dihydrogen anion ( H2-) we find one unpaired electron in ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Chemical bonding molecular orbitals of h2 and he2. This problem has been solved. Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Construct the molecular orbital diagram for n2 and then identify the bond order. Draw the lewis structure of pf 3a how many share. Relative AO Energies in MO Diagrams Use AO energies to draw MO diagram to scale (more or less). H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -19.4 eV -15.8 eV -32.4 eV -10.7 eV
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding ... LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital Similar phase of electron density (no node) adds together constructively. energy of isolated atoms bond order (H2 molecule) = (2) - (0) 2 = 1 bond 1sb H H H H σ∗ = 1s H H a - 1sb = antibonding MO = LCAO = linear combination of atomic orbitals node = zero ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Diatomic Species | MO theory | ChemogenesisChemical bonding - Molecular orbitals of H2 ... Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi...
Mo Diagram H2. molecular orbital diagram a molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory marcus va 100 primary volts 120 240 secondary volts 12 24. Construct The Molecular Orbital Diagram For H2- And Then Identify The Bond Order.
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
Chapter 1 Molecular Orbital Concepts A Concepts Of Mo Theory 1 Strong Covalent Bonds Consider The Pi Bond Of Ethene In Simple Molecular Orbital Terms The Qualitative Results Would Be The Same For Any Pi Or Sigma Bond Q The Overlap Of The Two
This photo about: Mo Diagram H2, entitled as Construct The Molecular Orbital Diagram For H2- And Then Identify Mo Diagram H2 - also describes Construct The Molecular Orbital Diagram For H2- And Then Identify and labeled as: mo diagramm aufstellen,mo diagramm cl2,mo diagramm i3,mo diagramm stickstoff,mo diagramm zeichnen, with resolution 3047px x 1556px
1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is .
Molecular Orbital Diagram Maker. These quizzes enable you to build your own molecular orbital diagram from components. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons.
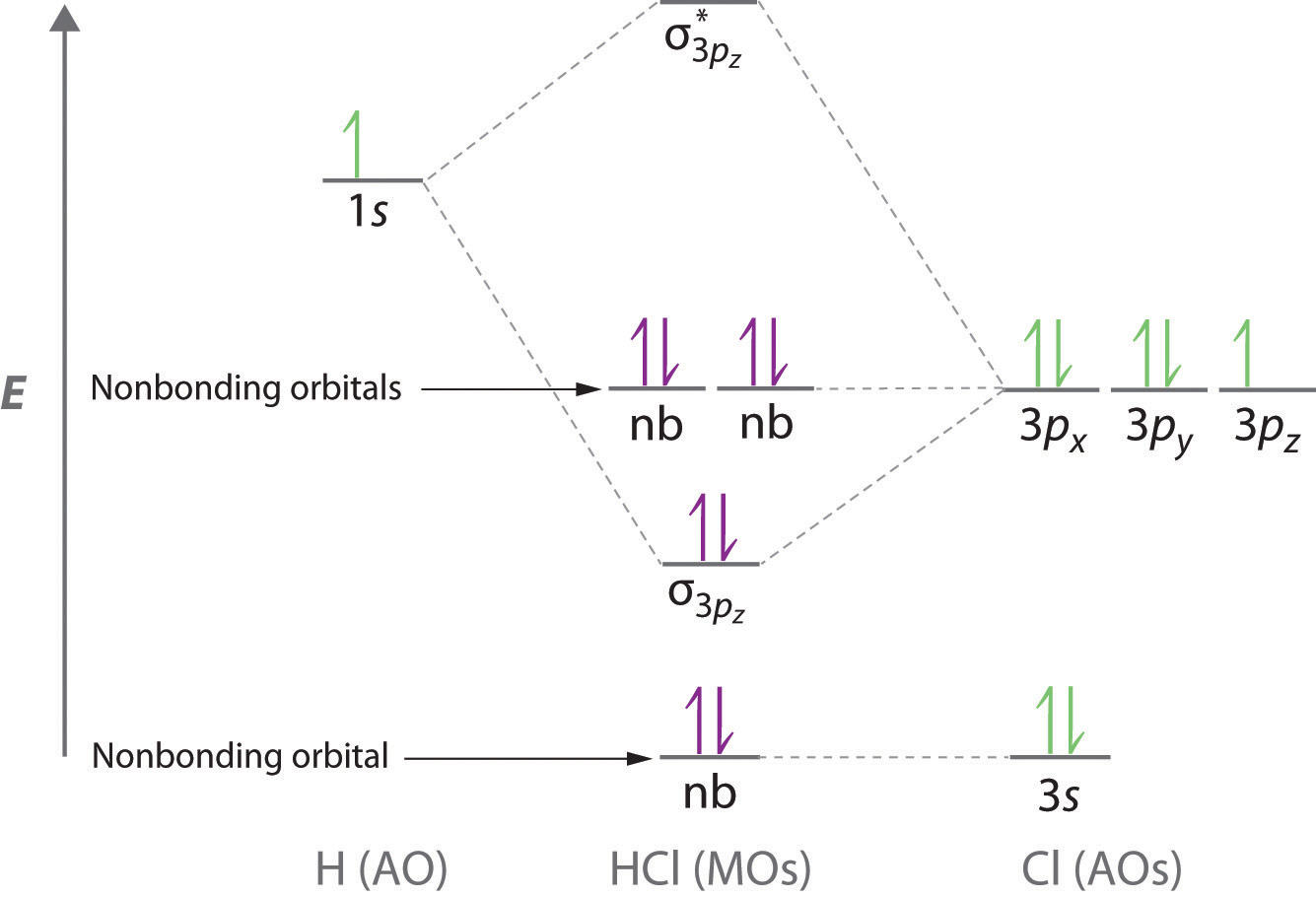
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
$$\ce{Be + H2 <=> BeH2}$$ The MO for $\ce{H2}$, which is shown in the figure below is taken from Wikipedia. The right side of the diagram you showed neither represents a hydrogen molecule, nor two independent (and hence equivalent) hydrogen atoms.
Paste a copy of each of your orbitals in the appropriate position in this diagram, as is shown in the MO diagram for H2 in Figure 5.45, compare (a) and (b) on page 225. Use the Aufbau Principle and Hund's Rule to place the valence electrons for N2 into these orbitals.
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
2) Draw a MO diagram for H2, including labeling for all orbital energies 3) Which of the following will have the strongest bond, on the basis of MO theory (S2, S2-, S2+). Provide an explanation ...
1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. σ bonding MO that is lower in energy than the constituent 1s AOs and an antibonding σ* MO that is at a higher energy than the 1s AOs.[1] Each.
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
Draw mo energy diagrams for the molecular ions h2 and h2. Construct the molecular orbital diagram for h2. When the 1s wave functions of the two ceh atoms are linearly combined we get a sigma s bonding orbital denoted as s 1s in the diagram herethis approach is called linear combination of atomic orbitals lcao.
The energy curves for ψ + and ψ-reveal the following properties of the ion H 2 +. The curve for ψ + refers to the ground state of the molecule where a minimum energy is found for a nuclear distance of approximately 2a o (i.e. 100pm). Thus, H 2 + should exist as a stable molecule. The calculated bonding energy is 1.77 eV. This is a quite satisfying result; from experiments we get 2.77 eV.
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds

Molecular Orbital Diagram Atomic Orbital Molecule Molecular Orbital Theory Mermaid Angle Text Triangle Png Pngwing
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds




















0 Response to "41 mo diagram for h2"
Post a Comment