41 miscibility gap phase diagram
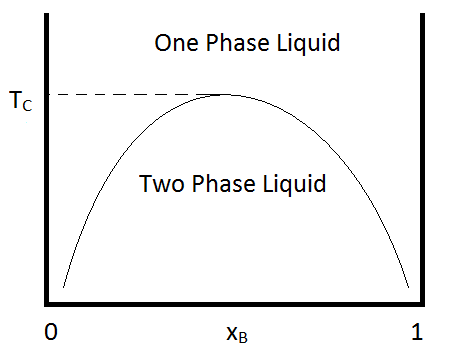
The miscibility gap between metals and oxides; Basicity as a concept and influence on viscosity; Chemical behaviour of slag depending on basicity; 11.30: Matte: structure and basic phase diagrams. The miscibility gap between metals and sulphides; The structure of matte; 12.30: Sandwich lunch; 13.30: Different elements and their tendencies The phenomenon is called a miscibility gap, where it seems that two liquids go together but only so far. Data on methanol-heptane were not available, but a phase diagram of methanol-cyclohexane is shown below: The x-axis goes from zero methanol (i.e., all cyclohexane) to all methanol (no cyclohexane). The large central area is the two-phase region.
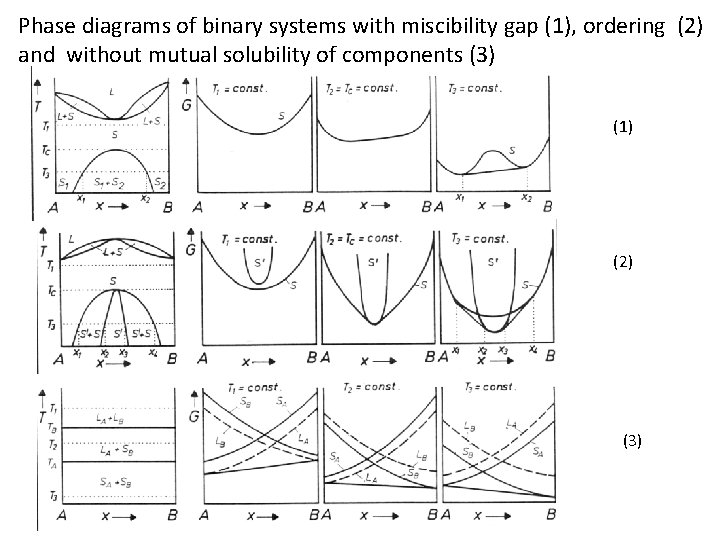
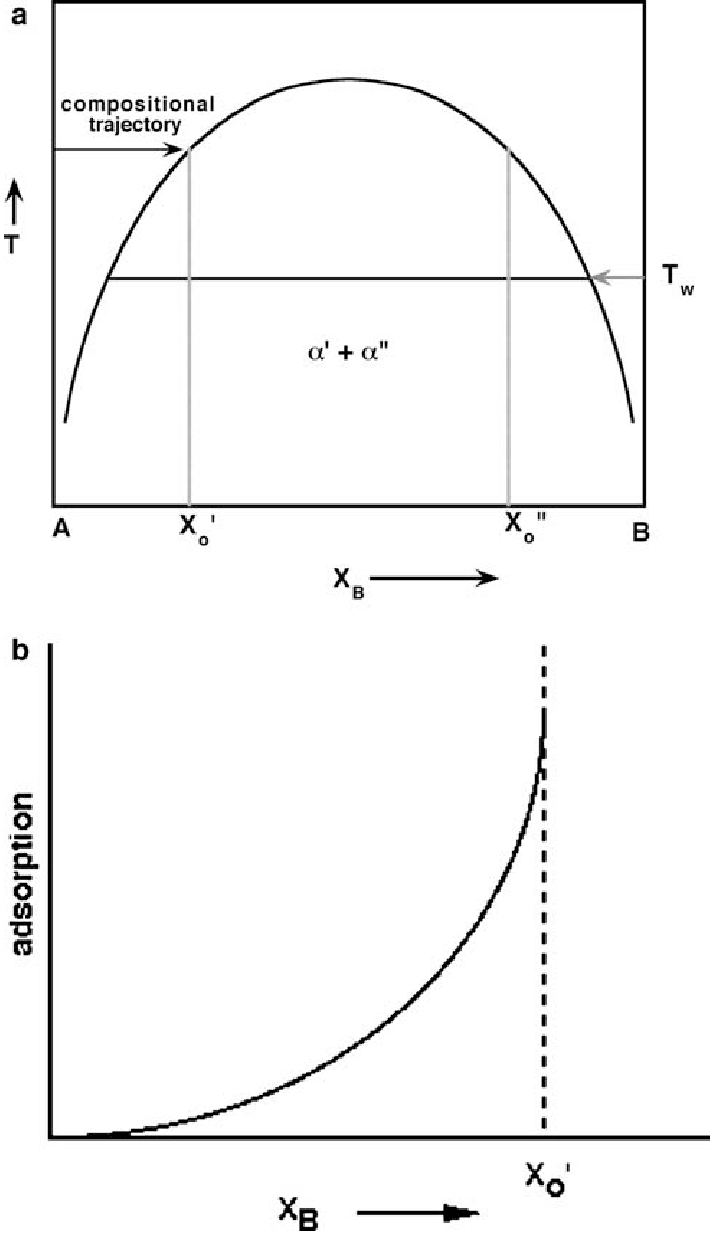
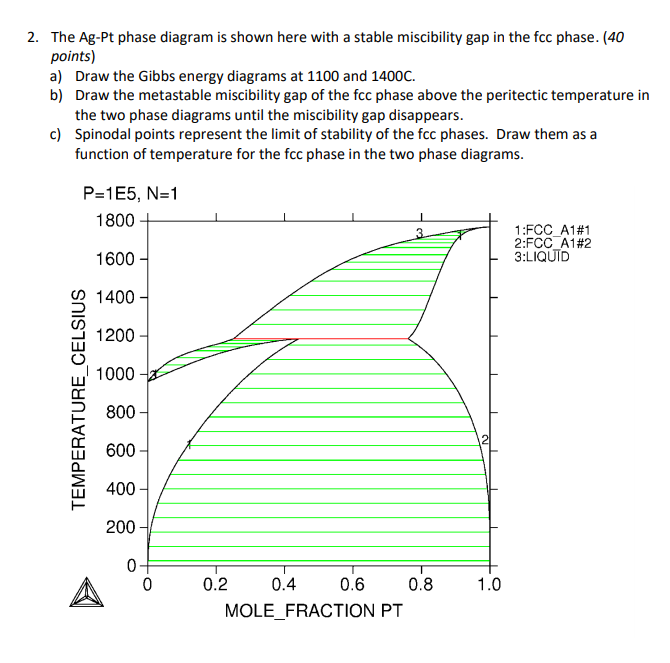
Draw a phase diagram with a miscibility gap. Then draw G vs. X for a particular temperature that cuts through the miscibility gap. At that temperature, then draw the G' vs. X and G'' vs. X curves. (where ' and '' are first and second derivatives respectively) Using these curves, show the position of spinodal points.
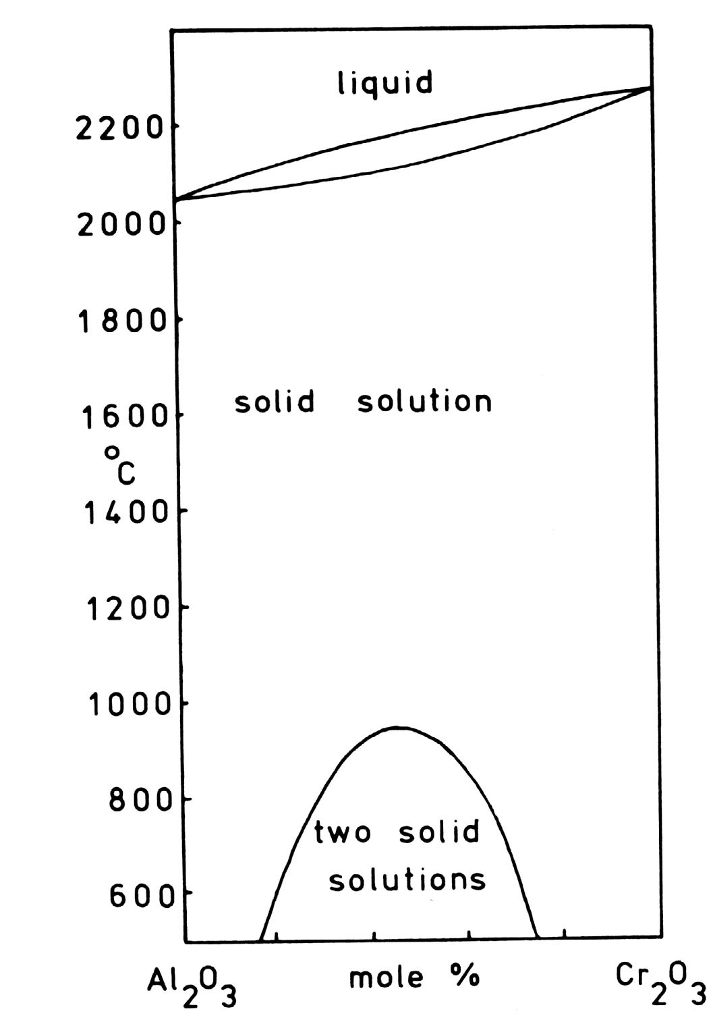
Miscibility gap phase diagram
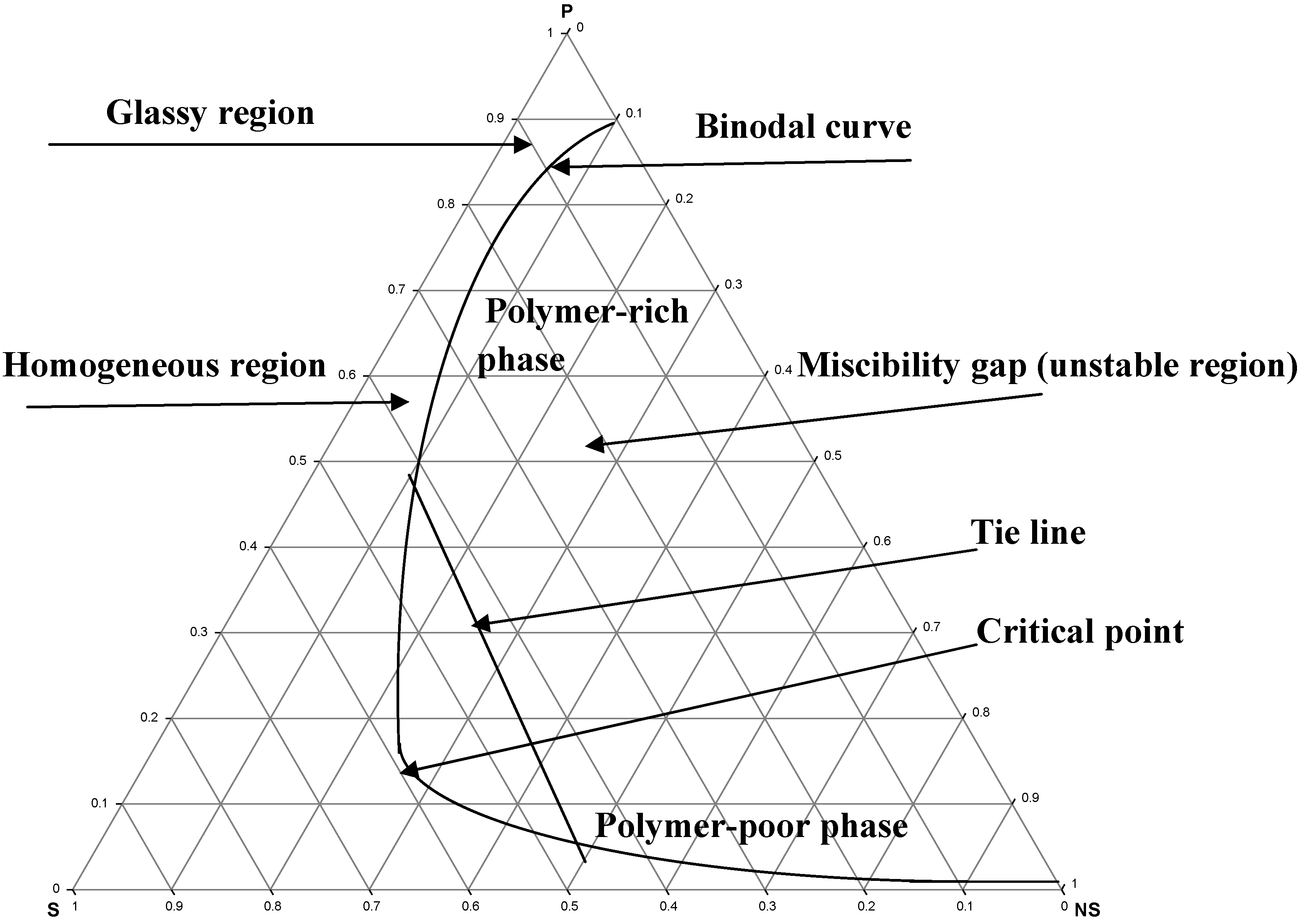
DOE PAGES Journal Article: Using phase boundary mapping to resolve discrepancies in the Mg 2 Si-Mg 2 Sn miscibility gap. This content will become publicly available on Fri Feb 18 00:00:00 EST 2022. Using phase boundary mapping to resolve discrepancies in the Mg ... The tie lines and the miscibility gap are strongly influenced by changes in temperature. A simplified representation of the phase equilibrium is the distribution diagram (Fig. 2.7). As demonstrated, the distribution equilibrium curve can be developed out of the triangular diagram. As generally reported in the literature, a high polymer precipitation rate often leads to an asymmetric membrane morphology with macrovoids and/or finger-like cavities beneath the skin layer [27,28] and, in some cases, researchers tended to associate phase diagrams that had a small miscibility gap (the distance between binodal curve and polymer ...
Miscibility gap phase diagram. Miscibility gap and possible intrinsic Griffiths phase in Sr (Fe 1 − x Mn x) 2 As 2 crystals grown by transition metal arsenide flux Long Chen, Cheng Cao, Hongxiang Chen, Jiangang Guo, Jie Ma, Jiangping Hu, and Gang Wang Phys. Rev. B 103, 134509 (2021) - Published 9 April 2021 Show Abstract A miscibility gap with x ranging from 0.4362(4) to 0.9612(9) is found in the Sr (Fe 1 − x Mn x) As 2 system—for x < 0.4362 (4), the phase remains in the parent tetragonal structure [space group I 4 / m m m (No. 139)], whereas for x > 0.9612 (9), the phase exhibits a trigonal structure [space group P − 3 m 1 (No. 164)]. [Phase diagram](http://i.imgur.com/dm26qae.png) One question is: Researchers claimed to have found a Pb-Sn-alloy with a weight% of 90 % Pb. Can this be true, according to the following phase diagram? Explain why. If I look at this phase diagram at 90% Pb I can see, that there are four different regions depending on the temperature. Starting from the top: Liquid (Melt), Liquid + Pb(s), Pb(s) and Pb(s) + Sn(s). But how can I now if the Pb(s) + Sn(s) region is a mixed crystal and when it is a m... In this "how to read a phase diagram" course the main focus is on the practical aspects of reading. It gives an insight into the type of information you can retrieve from a phase diagram and how to give an interpretation to the different lines and areas in the graph. ... Miscibility gap; 15.45: Opportunity for discussion; Aim. After the ...
The general phase separation (either by demixing or crystallization) can happen if a blend endures a thermal stress over T g and does not necessarily to get into the miscibility gap. A crystallization-induced phase separation is expected above film T g and outside the miscibility gap, which decouples crystallization from demixing. Such ... In the partial miscibility range, the phase behavior exhibits a miscibility gap. It shows phase separation during heat treatments, which is characterized by a lower segregation temperature. If one is below this temperature, only one phase is formed, and the substances are completely miscible with each other in all concentration ratios [ 2 ]. Samples treated in the miscibility gap showed an agreement of the Si-rich binodal line with calculated phase diagrams. However, the Sn-rich binodal line stays undefined, perhaps due to grain boundary pinning of diffusing atoms. The study elucidates the possibility of tailoring the microstructure of magnesium silicide-stannide alloys utilizing ... New miscibility gap alloys with a ceramic matrix have been explored in the ZrO 2 -Al, AlN-Al, AlN-(Al-Si), Al 2 O 3 -Al and MgO-Al systems with a view to creating oxidation-resistant macroscopically solid, phase change-enhanced, thermal energy storage materials. Materials were manufactured by mixing the components, pressing and firing at 700 °C under argon.
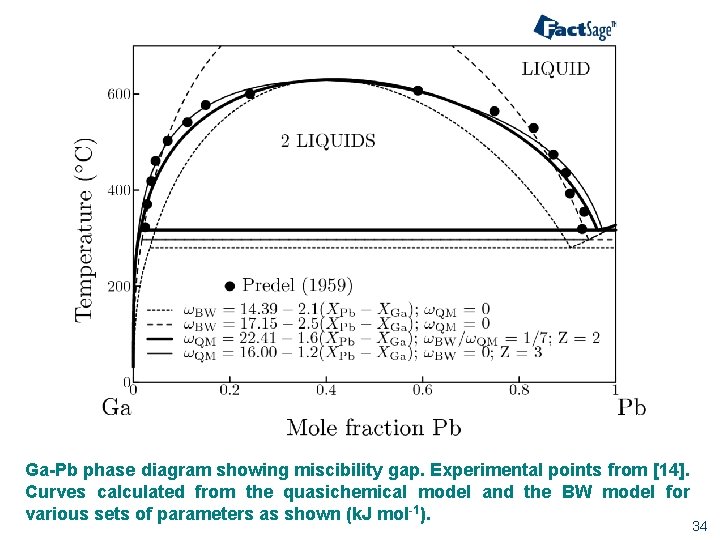
A eutectic phase diagram is found if the Gibbs potential of the solid phase with a miscibility gap becomes larger than the Gibbs potential of the liquid phase. The relation between the eutectic phase diagram and the g-plots for several temperatures is shown in Fig. 2.13. The phase diagram of the SnO 2-SiO 2 system was investigated using sealed Pt crucibles. A eutectic reaction of L → SiO 2 (s) + SnO 2 (s) was experimentally determined at about 1670 °C and 1.15 mol % SiO 2.No mutual solid solution between SiO 2 and SnO 2 was found. To explain this eutectic, a large liquid-liquid miscibility gap with a monotectic at about 1821 °C must exist between SnO 2 and ... Figure 1 shows a typical phase diagram of a two-component solution system exhibiting a miscibility gap (figure 1). The phase diagram is divided into regions showing one-phase stable and two-phase metastable and unstable phases. The binodal curve separates the stable homogenous phase from the two-phase regions, whereas the spinodal curve divides ... with Ref. 5, is the prediction of phase separation in the Ni-rich side of the phase diagram. The NC system is therefore predicted to be a miscibility gap system, with Co solubility of about five percent. This prediction is discussed, in section IV, in the light of previous experimental and theoretical results for this material. The predicted
In the case of the Ir-Pt system, the (111) peak splits into two peaks. This is due to the coexistence of two phases and implies that Ir and Pt are not completely mixed, which is consistent with the large miscibility gap of the Ir-Pt phase diagram. Conclusion
Na2S-S phase diagram of Pearson and Robinson was revised in the Na2S2-S range and extended to show the Na-rich border of the miscibility gap. So far very little has been known about the electro- chemistry of the Na-S system in the molten state. With the application of E-alumina and related struc-
Figure 2(a)-(c) occur inside 2-phase miscibility gaps, while the central portion of Fig. 2(d) only occurs inside a 3-phase miscibility gap. It contains a cone point in the spinodal surface,[15,16,17] which can have a significant influence on how HEAs are designed, and heat treated to optimize properties.The contour lines outside the central triangle in Fig. 2(d) have the same shape as a ...
The general phase separation (either by demixing or crystallization) can happen if a blend endures a thermal stress over T g and does not necessarily to get into the miscibility gap. A ...
With increasing pressure and temperature in Earth's interior, the miscibility gap between silicate melt and aqueous fluid diminishes, and single phase supercritical fluid with an intermediate composition (both silicate solute and H 2 O are in the range of 30-70 wt. %) can form, most likely in deep subduction zones (Manning, 2004. Manning, C.E. (2004) The chemistry of subduction zone fluids.
The reason radical change occurs in the solubility of thermoresponsive polymers when the temperature changes is that a miscibility gap is observed in the phase diagram [72,73].
compositions were selected as they spanned the miscibility gap in predicted ternary phase diagrams, as shown in Figure 1 . Each alloy was melted under an inert Ar atmosphere,
According to the pseudo-binary phase diagram (see Fig. 5a), there exists a miscibility gap in the ternary InGaP compound, which makes it challenging for the composition tunability in InGaP nanowires. Using thermo-calc software, we calculate the solidification of InGaP from supersaturated Au droplet to simulate the Au-seeded nanowire growth.
On the indium rich side of the phase diagram a wide miscibility gap up to temperatures higher than 1400 K is found, which results in phase separated layers. The experimentally observed indium solubilities in the PLD samples are in the range of x = 0.45 and x = 0.55 for monoclinic and hexagonal single-phase films, ...
@article{osti_1821155, title = {Revealing the Phase Separation Behavior of Thermodynamically Immiscible Elements in a Nanoparticle}, author = {Chen, Peng-Cheng and Gao, Mengyu and Yu, Sunmoon and Jin, Jianbo and Song, Chengyu and Salmeron, Miquel and Scott, Mary C. and Yang, Peidong}, abstractNote = {Phase-separation is commonly observed in multimetallic nanomaterials, yet it is not well ...
View Handout_20201119_PhaseDiagram_AuNi.pdf from MSNE 503 at Rice University. Phase diagram with a miscibility gap
In previous studies [18,19], the phase behavior of a binary P3HT/PC 61 BM blend was elucidated, leading to temperature-composition phase diagrams . Then the binary low bandgap conjugated polymer solutions and blends were investigated, resulting in various phase diagrams as a function of solvent species, polymer, and chain length . In this study ...
The eutectic or solid-miscibility-gap alloys belong to group IV (G E ≥0). The Au-Ni alloy has a solid-miscibility gap and therefore belongs to group IV. The Au-Ni melt is in the region defined by V E >0 and G E >0, in agreement with the correlation proposed by Iida and Guthrie . Download : Download high-res image (371KB)
As generally reported in the literature, a high polymer precipitation rate often leads to an asymmetric membrane morphology with macrovoids and/or finger-like cavities beneath the skin layer [27,28] and, in some cases, researchers tended to associate phase diagrams that had a small miscibility gap (the distance between binodal curve and polymer ...
The tie lines and the miscibility gap are strongly influenced by changes in temperature. A simplified representation of the phase equilibrium is the distribution diagram (Fig. 2.7). As demonstrated, the distribution equilibrium curve can be developed out of the triangular diagram.
DOE PAGES Journal Article: Using phase boundary mapping to resolve discrepancies in the Mg 2 Si-Mg 2 Sn miscibility gap. This content will become publicly available on Fri Feb 18 00:00:00 EST 2022. Using phase boundary mapping to resolve discrepancies in the Mg ...
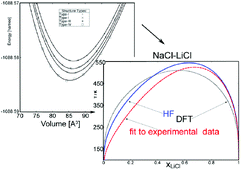
Ab Initio Computation Of Low Temperature Phase Diagrams Exhibiting Miscibility Gaps Physical Chemistry Chemical Physics Rsc Publishing


















0 Response to "41 miscibility gap phase diagram"
Post a Comment