41 construct the orbital diagram for ni
Construct the orbital diagram for ni. Construct the orbital diagram for ni. These are simplified diagrams of how electrons are arranged within the orbitals for a. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. Draw the orbital diagram for ion co 2. 06.11.2021 · The photogenerated electrons on the LUMO orbital reduce S 2 O 8 2-to SO 4 2-in solution, while the photogenerated holes gathered on the HOMO orbital oxidize water to produce oxygen. . The speculated mechanism of the photocatalytic H 2 production by water reduction reaction in EY-sensitized system was illustrated in Fig. 7b. The defective Cu-BTC ...
Question: Construct the orbital diagram for Ni. This problem has been solved! See the answer ...

Construct the orbital diagram for ni
01.06.2021 · Some representative building blocks used to construct COFs. COFs, covalent organic frameworks. Despite of the growing number of the developed architectures, novel design strategies for efficient luminescent COFs are still highly desirable. This review will summarize the recent advances on photoluminescent COFs from the mechanisms to the proposed design strategies and finally to the ... Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund’s Rule: Given sev... Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2.
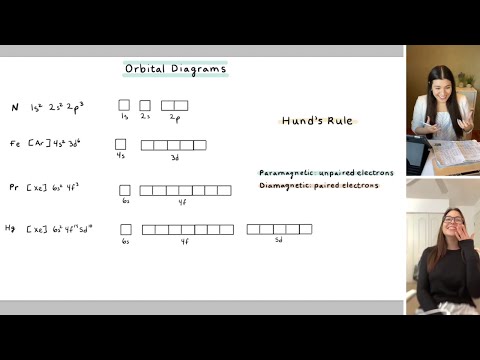
Construct the orbital diagram for ni. The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ... 1x 1.25x 1.5x 1.75x 2x. FREE Expert Solution. We’re being asked to construct the orbital diagram for N3–. For this problem, we need to do the following: Step 1: Determine the electron configuration of the neutral element. Step 2: Determine the electron configuration of the ion. Step 3: Construct the orbital diagram for the ion. 82% (439 ... Academia.edu is a platform for academics to share research papers. Question: Construct the orbital diagram for Ni. Construct the orbital diagram for Ni. see more. Best answer. Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged within the orbitals for a. Answer to Construct the orbital diagram for Ni. Start by adding the appropriate ...
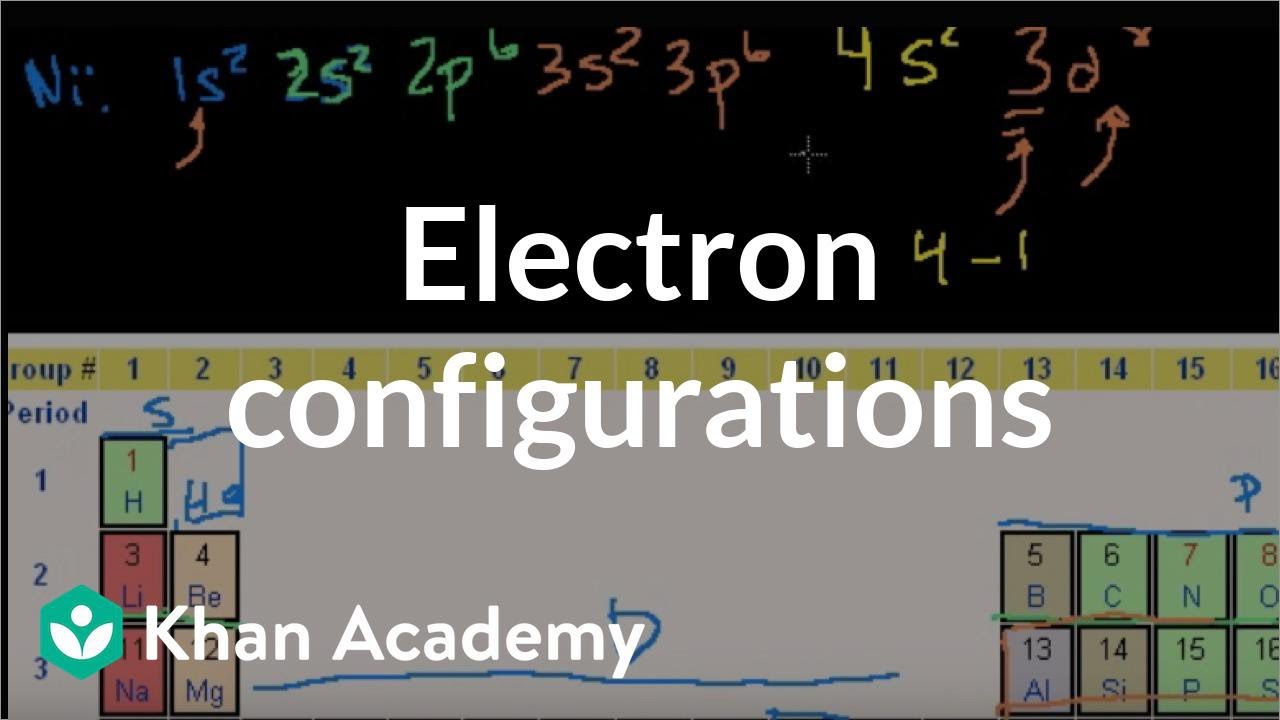
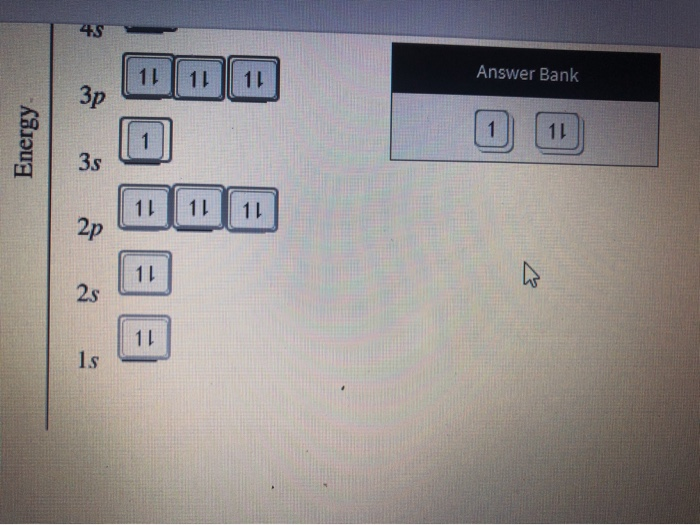
Problem: Construct the orbital diagram for Ni. FREE Expert Solution. Ni → atomic # 28 → 28 electrons. Ni will pass through 1s, 2s, 2p, 3s, 3p, 4s2, 3d. Following Aufbau principle (fill lowest energy first) and Hund's rule (half-filled first before totally filled) 82% (205 ratings) All right, so here, we to construct an orbital diagram for nickel. Okay, let's go ahead and write its full electron configuration first.27 Aug 20181 answer · Top answer: We’re being asked to construct the orbital diagram for Ni. For that, we first need to determine the electron configuration of Ni.Recall that for a ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Construct the orbital diagram for nickel. Answer Bank 111 Energy. Sigma pi bond formation Orbital overlap concept ncert
26 Jan 2021 — Valence electrons are the electrons which are located in the outer shell or orbit. There are 28 electrons in the nickel in the 4 orbits and the ... Oxidation States, +2,3. Electrons Per Shell, 2 8 16 2. Electron Configuration, [Ar] 3d8 4s2. 1s2 2s2 2p6 3s2 3p6 3d8 4s2. Orbital Diagram. Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a way that's easy for you to ... Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2.
Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund’s Rule: Given sev...
01.06.2021 · Some representative building blocks used to construct COFs. COFs, covalent organic frameworks. Despite of the growing number of the developed architectures, novel design strategies for efficient luminescent COFs are still highly desirable. This review will summarize the recent advances on photoluminescent COFs from the mechanisms to the proposed design strategies and finally to the ...

20 Draw The Orbital Notation Beyond The Noble Gas Core For Osmium 20 Draw The Orbital Notation Beyond The Noble Gas Core For Osmium Homeworklib
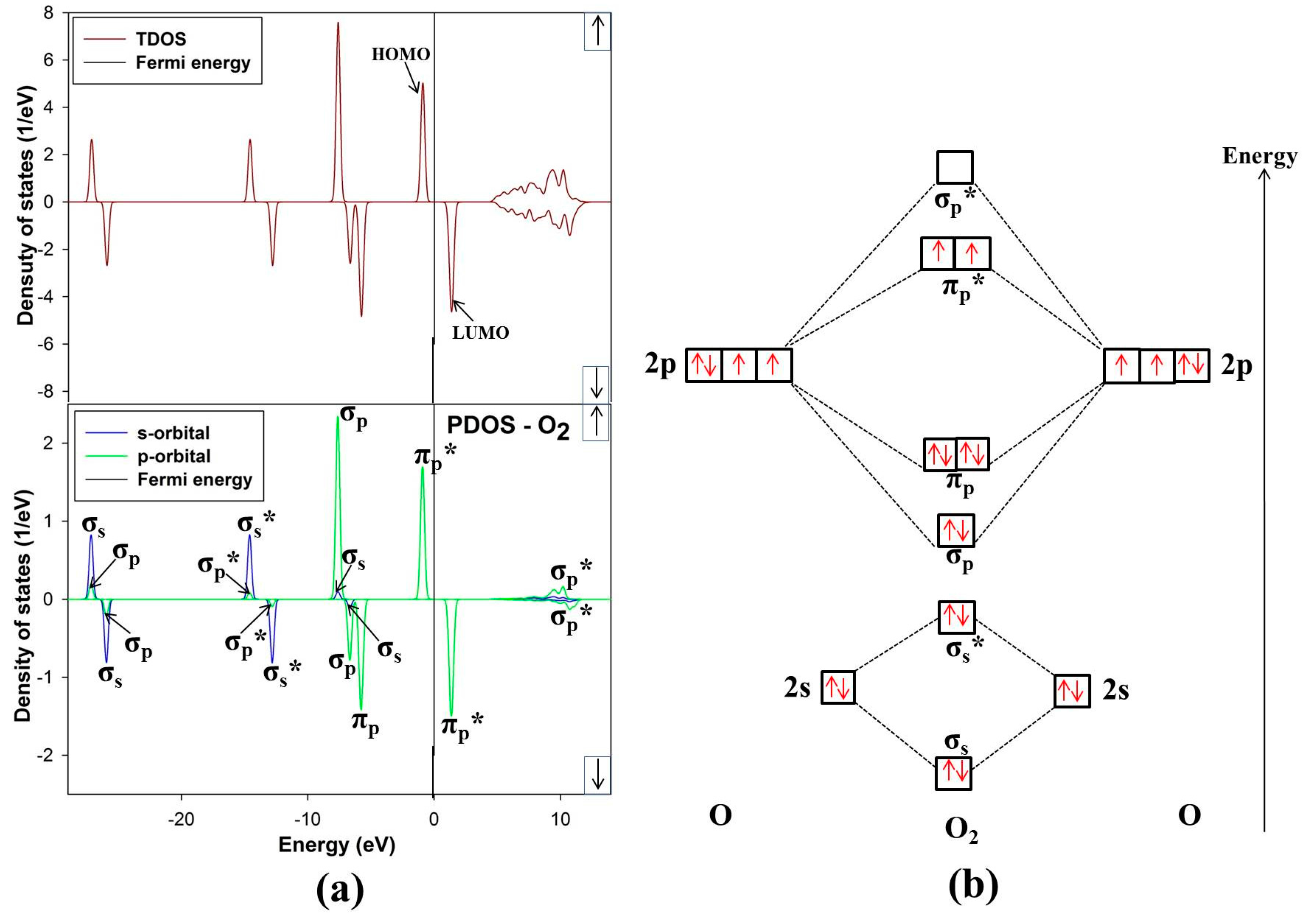
Minerals Free Full Text Ab Initio Studies Of O2 Adsorption On 110 Nickel Rich Pentlandite Fe4ni5s8 Mineral Surface Html
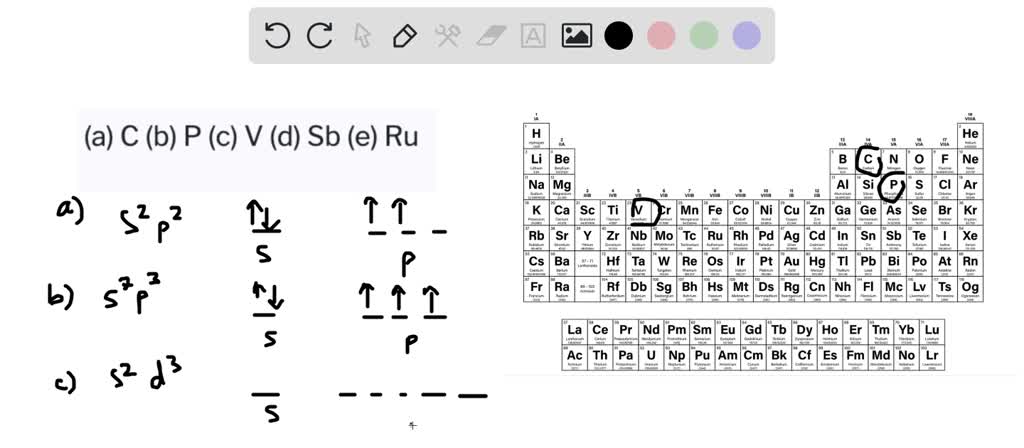
Solved Draw The Orbital Diagram For The Valence Shell Of Each Of The Following Atoms A C B P C V D Sb E Ru

1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2
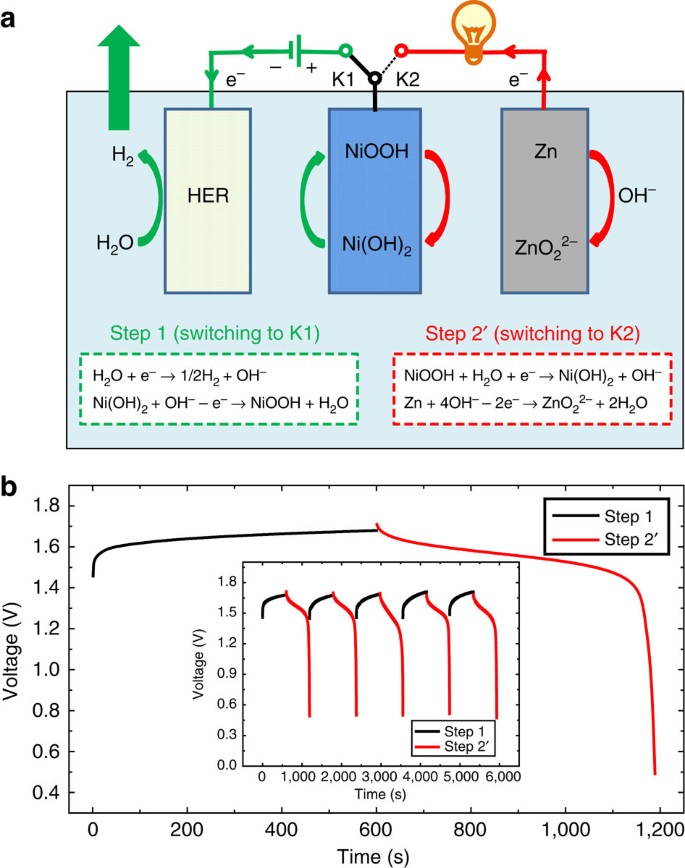
Separating Hydrogen And Oxygen Evolution In Alkaline Water Electrolysis Using Nickel Hydroxide Nature Communications
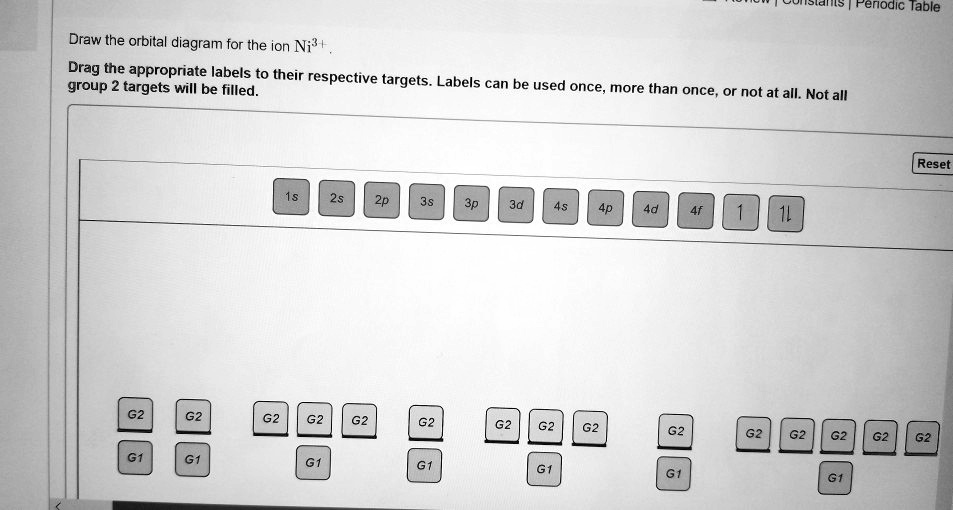
Solved Feriodic Lable Draw The Orbital Diagram For The Ion Ni Drag The Appropriate Labels To Their Respective Group 2 Targets Will Be Filled Targets Labels Can Be Used Once More Than Once

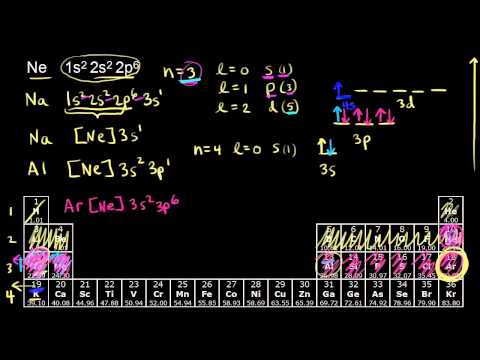











0 Response to "41 construct the orbital diagram for ni"
Post a Comment