40 reaction coordinate diagram endothermic vs exothermic
Exothermic reactions release energy to their surroundings, because the products are lower in energy than the reactants. You can think about this visually using a reaction energy diagram, as seen below: And endothermic reaction (left) and an exothermic reaction (right) plotted on a plot of energy against the reaction coordinate (a measure of the ... Energy diagrams are also referred to as reaction coordinate diagrams! ... Exothermic and Endothermic Reaction Energy Diagrams.
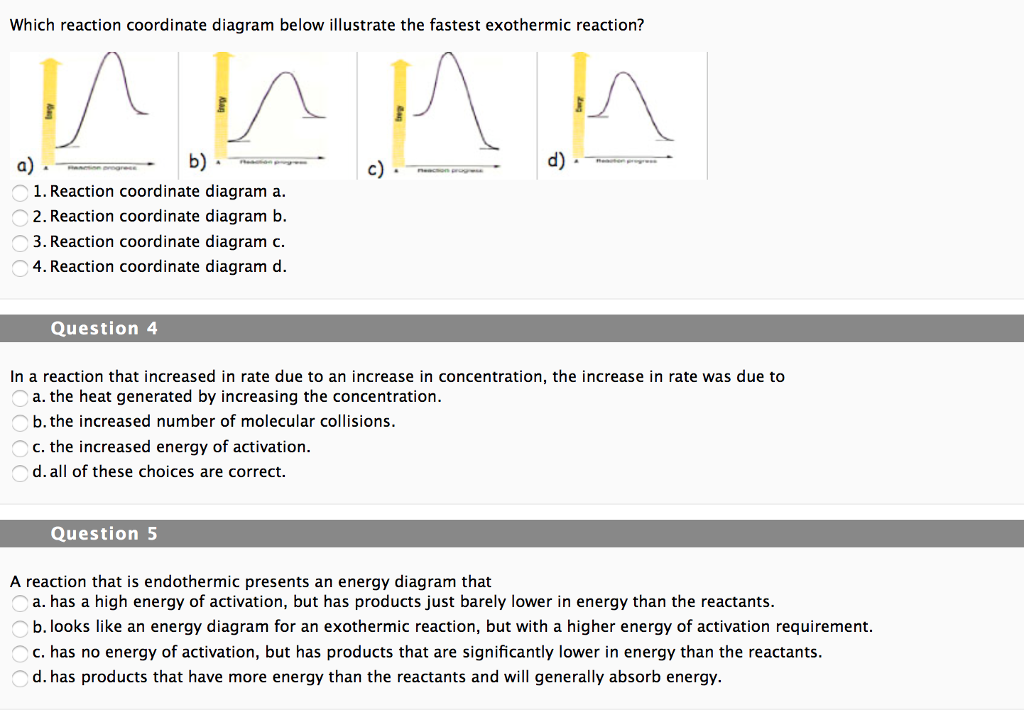
Science. Chemistry. Chemistry questions and answers. draw an energy-reaction coordinate diagram for both exothermic and endothermic reactions and compare the two. how would temperature changes observed using a calorimeter differ from exothermic and endothermic reactions?

Reaction coordinate diagram endothermic vs exothermic
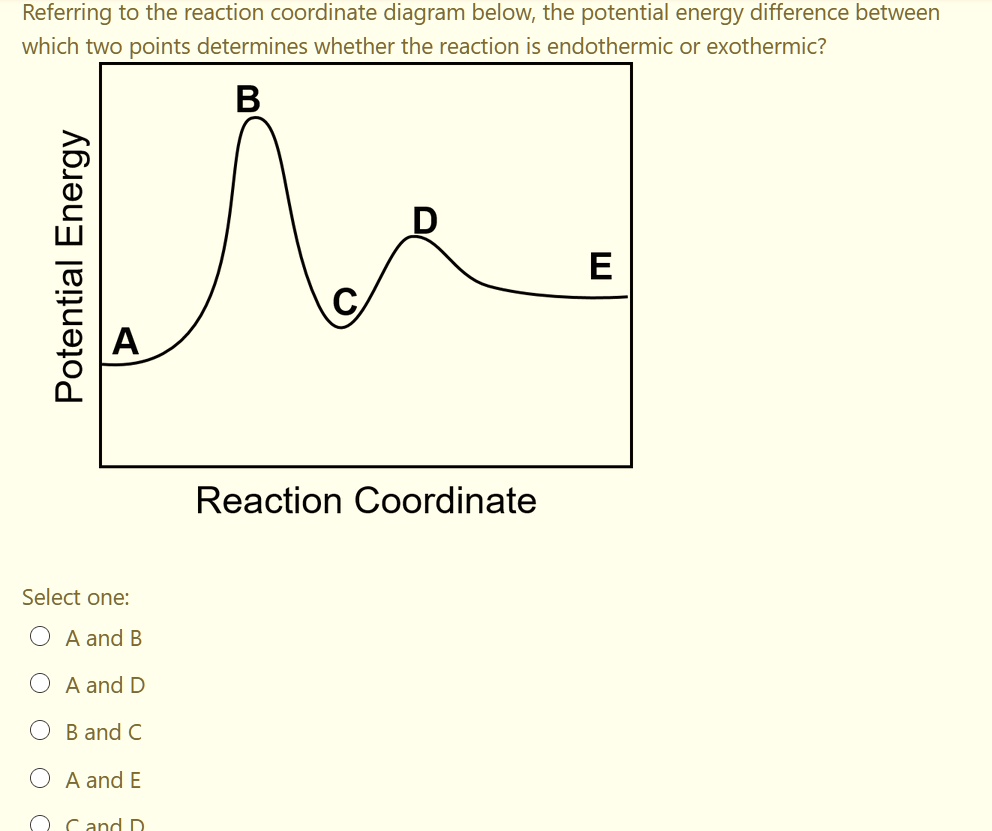
Endothermic Reactions vs. Exothermic Reactions. An Exothermic system, where Exo means outside, releases thermal energy into its surroundings when it proceeds. This energy is created when the bonds ... c) Draw an energy level diagram for the reaction. 5. Draw an energy level diagram for a reaction in which the total energy of the reactants is 50 kJ mol-1, the total energy of products is 120 kJ mol-1 and the activation energy for the forward reaction is 120 kJ mol-1. Label the diagram clearly. Is this reaction exothermic or endothermic? С B D Potential Energy A E Reaction Coordinate Endothermic Exothermic Question : Does the following reaction coordinate diagram correspond to an endothermic or exothermic process? This problem has been solved!
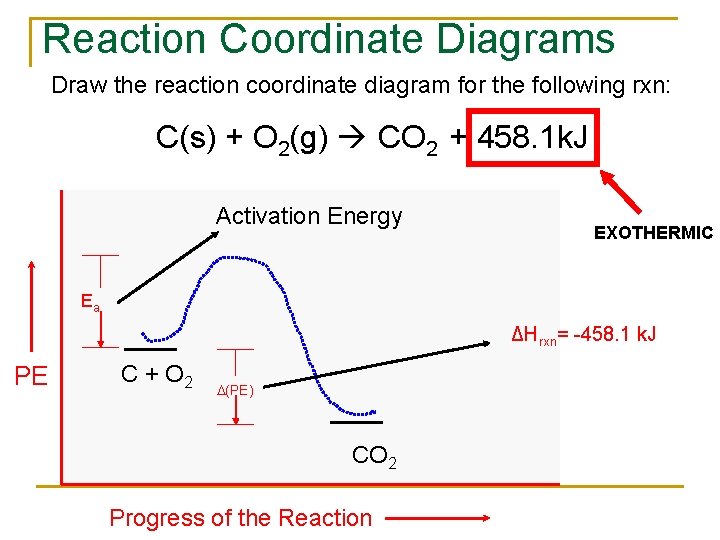
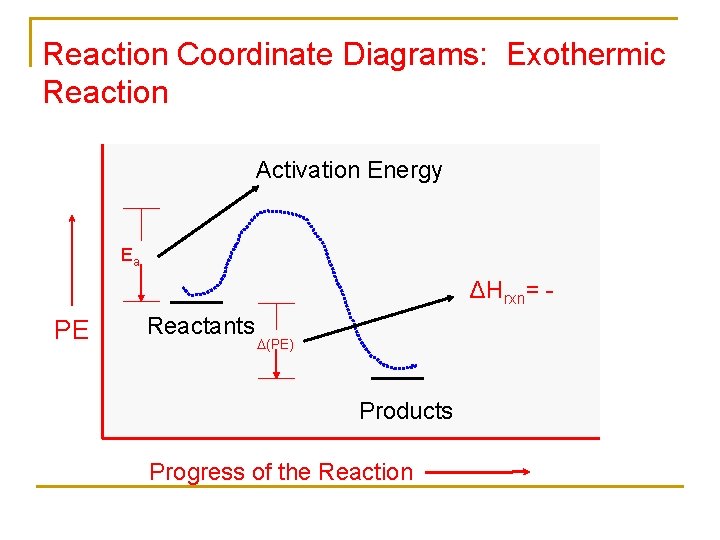
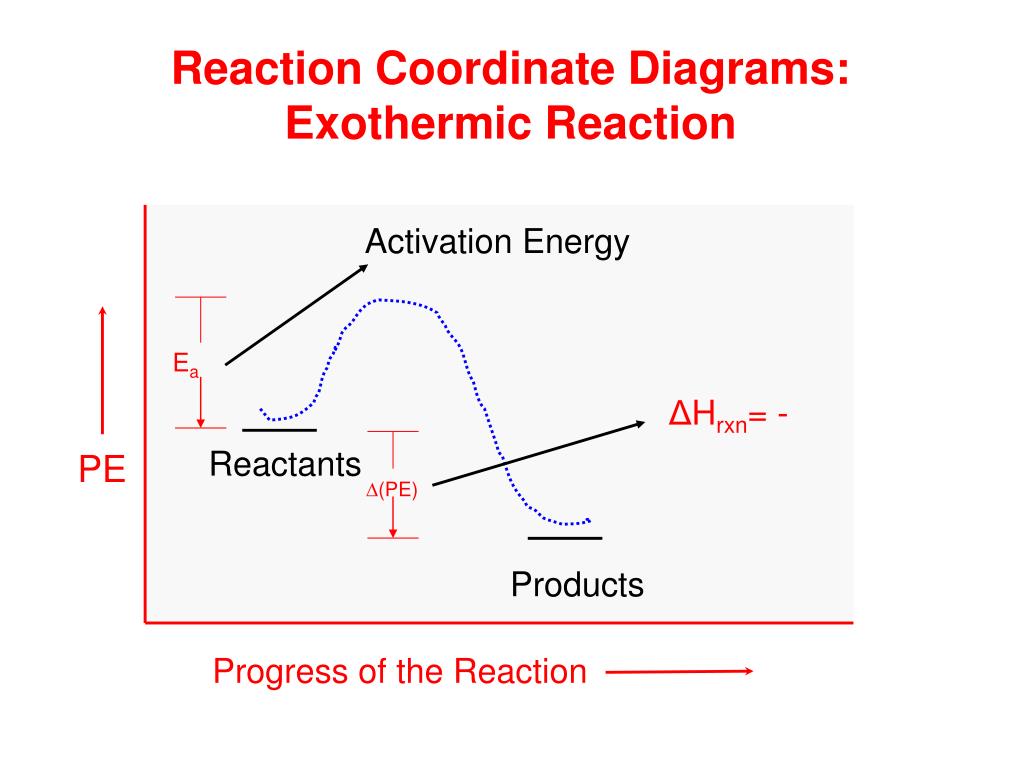
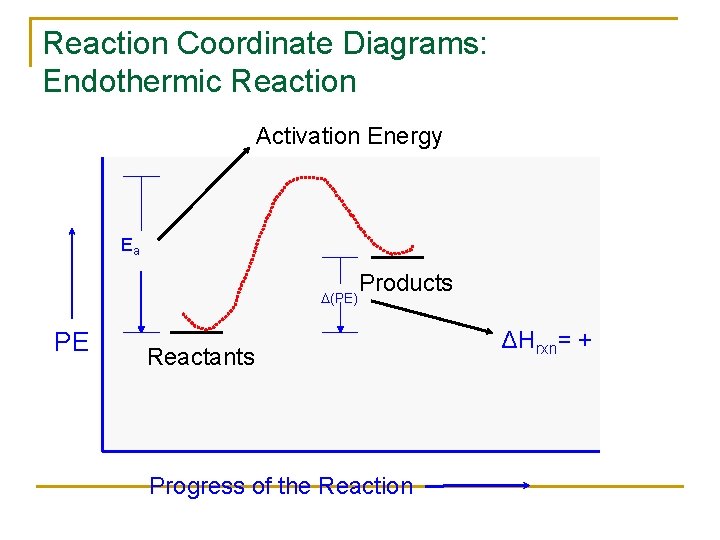
Reaction coordinate diagram endothermic vs exothermic. Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 . Energy profile diagrams for endothermic and exothermic reactions every chemical substance has a certain amount of chemical energy. The energy level increases in an endothermic. An energy level diagram shows whether a reaction is exothermic or endothermic. Energy must be input in order to raise the particles up to the higher energy level. Endothermic Reaction Energy Diagram. bbc gcse bitesize energy level diagrams energy level diagrams during a chemical reaction energy is either taken in or given out during exothermic reactions energy is given out this means that the energy of the products will be lower than the energy of the reactants this can be represented using an energy level diagram endothermic vs exothermic reactions ... Note:As we understand the graph for the exothermic process similarly we have an endothermic reaction, in which we have to provide heat from the surrounding ...
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... This chemistry video tutorial focuses on endothermic and exothermic reactions. It explains the flow of heat energy into and out of the system and surroundin... ... tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Exothermic vs endothermic reaction graphs More Kinetics Links Potential Energy Diagrams A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram.Sometimes a teacher finds it necessary to ask questions about PE diagrams that ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction. Re: Endothermic and Exothermic Graphs. In both graphs, the x axis should indicate progress of a reaction and the y axis should indicate the energy of a reaction. In an exothermic graph, the products are at a lower energy level than the reactants, because energy is released. In an endothermic graph, the products are at a higher energy level than ...
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.”. Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic.
The Exothermic graph looks like this: The reason why the products are lower is beacuse Exo means out, so the reactants lose energy. The reaction Coordinate is just the run time. The Endothermic Graph looks like this: (Ignore the Delta E it's supposed to be Delta H) The reason why the products are higher is because Endo means in, so the ...
An example of an exothermic reaction is the chemical reaction between sodium and chlorine, which results in the formation of sodium chloride (also known as common salt). Endothermic Reactions The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat.
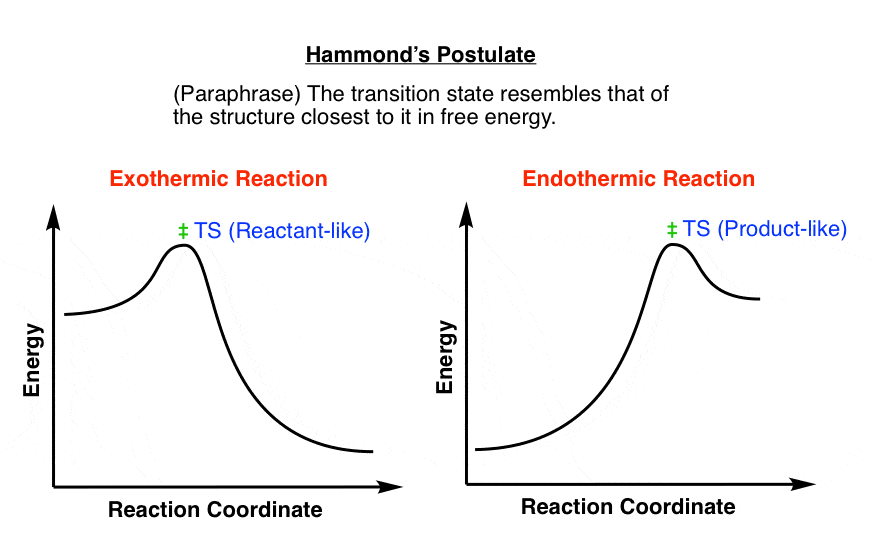
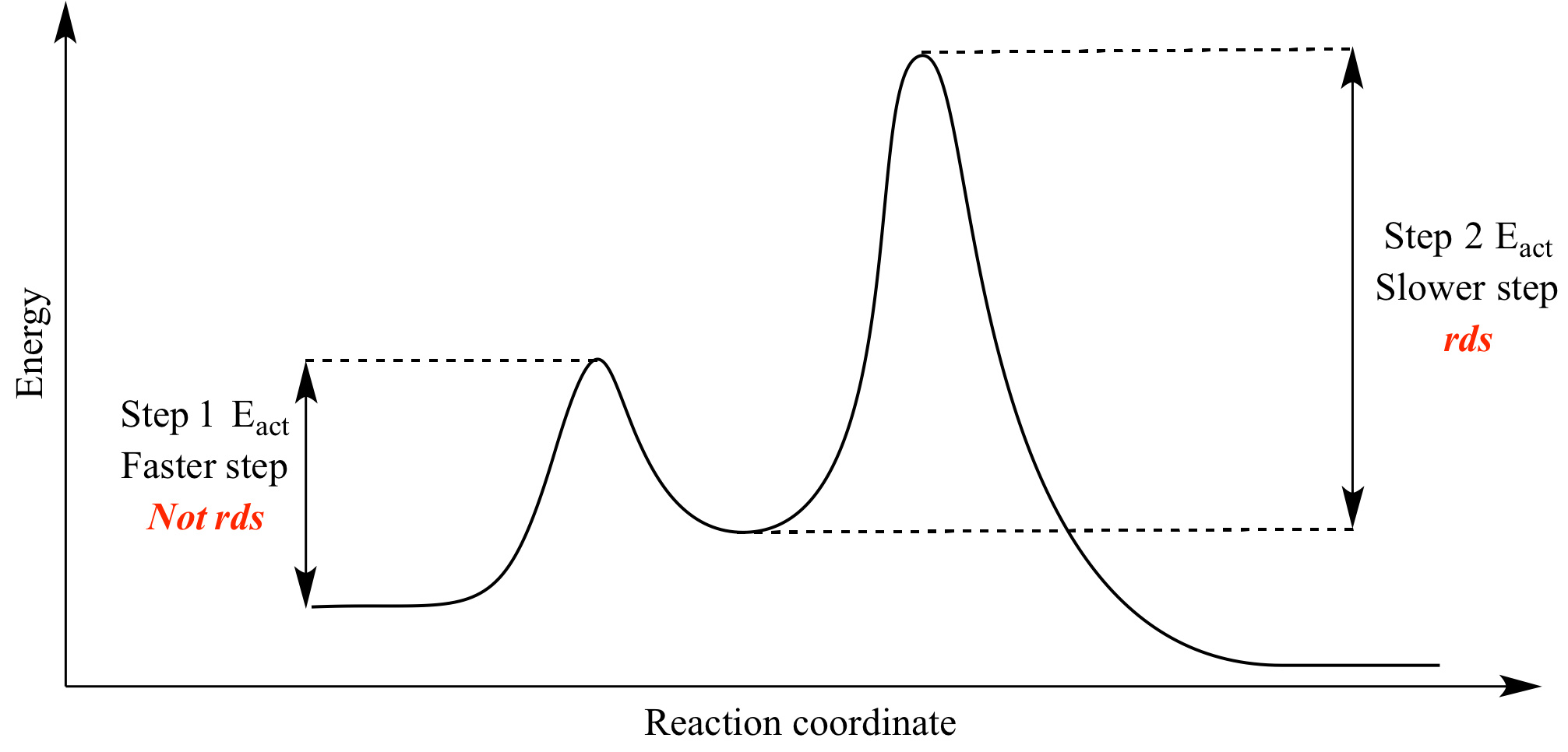
Secondly, activation energy (Ae) is usually higher for endothermic reactions than exothermic reactions so the 'distance' between the peak (energy level of ...
For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, ...
5.3. Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of ...
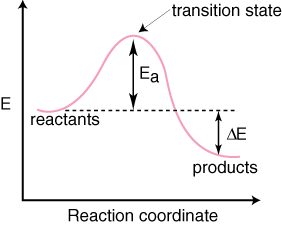
Thermodynamics vs Kinetics Overview A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be
In exothermicreactions, there is more energy in the reactants than in the products. In endothermicreactions, there is less energy in the reactants than in the products. Activation Energy. The activation energy in an exothermic reaction is energy at the top of the curve minus the energy of the reactants.
This is shown in an Energy vs Reaction Coordinate plot. useful in showing: 1) whether a reaction is an exo- or endothermic process, 2) which reaction Exothermic reactions will have products that are lower in energy than the reactants.Endothermic Reaction Energy Diagram - 62 Unique Figure Of Endothermic Reaction Energy Diagram, thermochemistry ...
The potential energy diagrams for the exothermic reaction ... difference between the diagrams for the exothermic vs. endothermic reactions? _____ Explain _____ ... Reaction Coordinate X + Y + energy XY (continued) 7. On the diagram below, draw a dotted line to show the pathway of a catalyzed reaction. Add the
An endothermic reaction has the heat term on the left side and an exothermic reaction has the heat term on the right side. Increasing the heat term shifts the reaction to the side without the term and decreasing the term shifts the reaction to the side with the term. Top. 5 posts • Page 1 of 1. Return to "Applying Le Chatelier's Principle ...

Activation Barrier To Reaction Process Kinetic Molecular Theory In A Reaction There Must Be A Chemical Changes Reactant Bonds Are Broken Product Bonds Ppt Download
Endothermic Vs Exothermic Reactions Article Khan Academy. Ch 10 Kinetic And Thermodynamic Control. Reaction Coordinate Diagrams College Chemistry ... Reactionprofiles. Changes In Temperature. Energy Reaction Coordinate Diagram Endothermic Vs Exothermic. Reaction Coordinate Diagram Sn2. Quiz Worksheet Energy Reaction Coordinate Diagrams Study ...
Draw the reaction coordinate diagram for an exothermic reaction. 7. The bonds between the atoms of the reactants/products need to be broken first, this is an endothermic / exothermic process.
This means that the reaction produces heat for the solution to absorb and q for the reaction is negative. When heat is absorbed from the solution q for the solution has a negative value. This means that the reaction absorbs heat fron the solution, the reaction is endothermic, and q for the reaction is positive. Top
A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change ... ΔE = q + w! ΔH = q p! •Most reactions are EXOTHERMIC and ΔGo ≈ Ho = (-)! • But, there are many ENDOTHERMIC reactions such as photosynthesis that occur.! •In this case, entropy is significant and must be
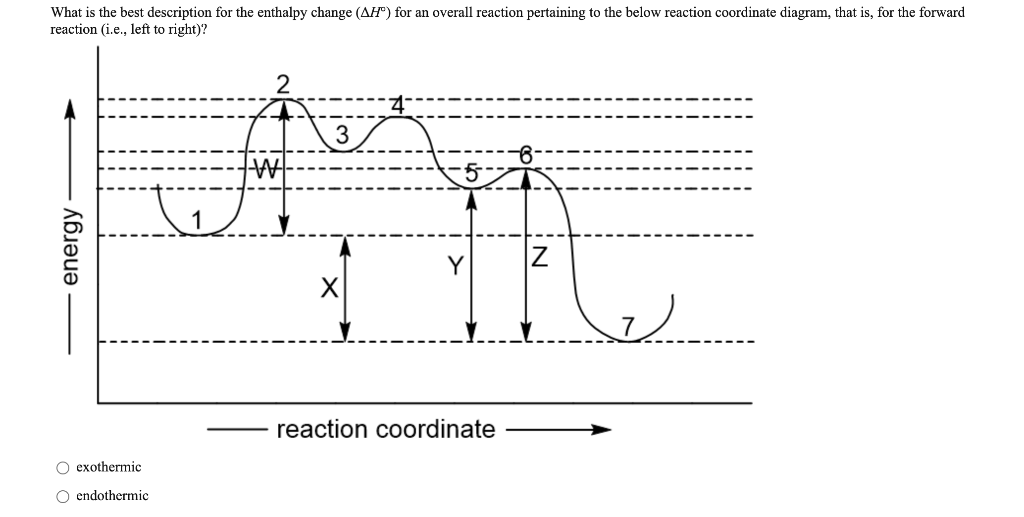
Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.
A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism.
A reaction can be endothermic or exothermic. If it is endothermic, then the reaction requires energy overall, so delta E is positive. On the graph, the products end up at a higher energy than the ...
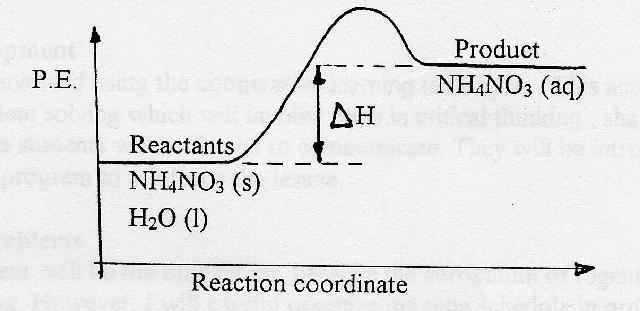
Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
Reaction Coordinate Diagram Endothermic. energy profile chemistry for a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single ener ic pathway along energy profiles chemistry tutorial aus e tute energy profiles or energy diagrams for endothermic and exothermic reactions with or without a catalyst tutorial with worked examples ...
What Is Difference Between Endothermic And Exothermic Reaction If Both Require Activation Energy Quora
С B D Potential Energy A E Reaction Coordinate Endothermic Exothermic Question : Does the following reaction coordinate diagram correspond to an endothermic or exothermic process? This problem has been solved!
c) Draw an energy level diagram for the reaction. 5. Draw an energy level diagram for a reaction in which the total energy of the reactants is 50 kJ mol-1, the total energy of products is 120 kJ mol-1 and the activation energy for the forward reaction is 120 kJ mol-1. Label the diagram clearly. Is this reaction exothermic or endothermic?
Endothermic Reactions vs. Exothermic Reactions. An Exothermic system, where Exo means outside, releases thermal energy into its surroundings when it proceeds. This energy is created when the bonds ...

















0 Response to "40 reaction coordinate diagram endothermic vs exothermic"
Post a Comment