39 orbital filling diagram for bromine
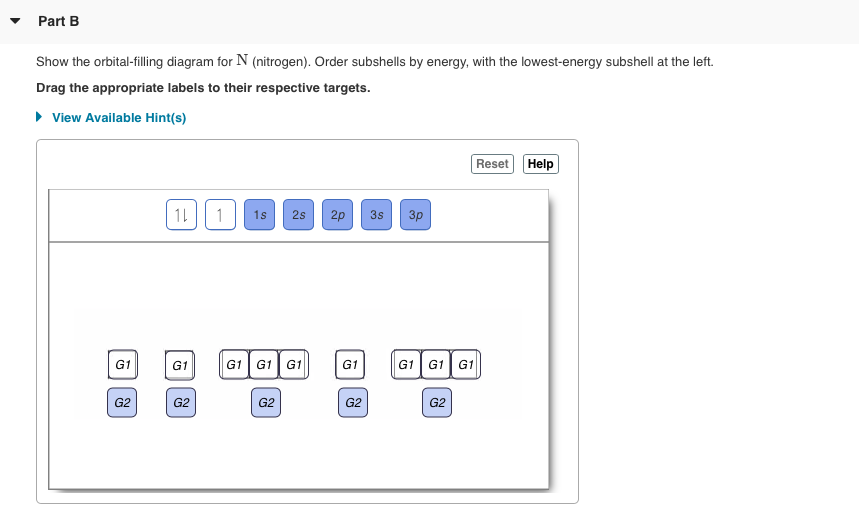
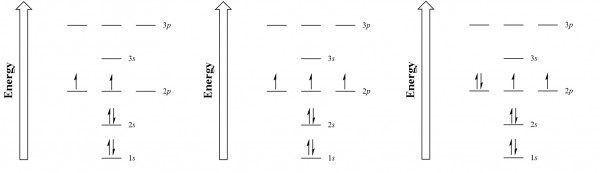
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal –each electron occupies the lowest energy orbital available; German for “build up” •Electrons are notated with an arrow –Up arrow goes first then, down arrow –Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top .Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top.
1 Feb 2021 — Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is ...
Orbital filling diagram for bromine
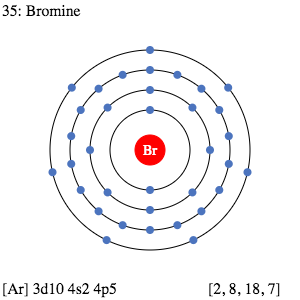
What is a pi bond describe with the aid of a diagram What is a pi bond describe with the aid of a diagram ... Oxidation States, ±1,+5. Electrons Per Shell, 2 8 18 7. Electron Configuration, [Ar] 4s2 3d10 4p5. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Orbital Diagram. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. • If you were to continue in that fashion, however, you would assign chromium and copper the following incorrect configurations. Determine the numbers of electrons which the atoms will lose and gain by applying the Octet Rule. AMU ...
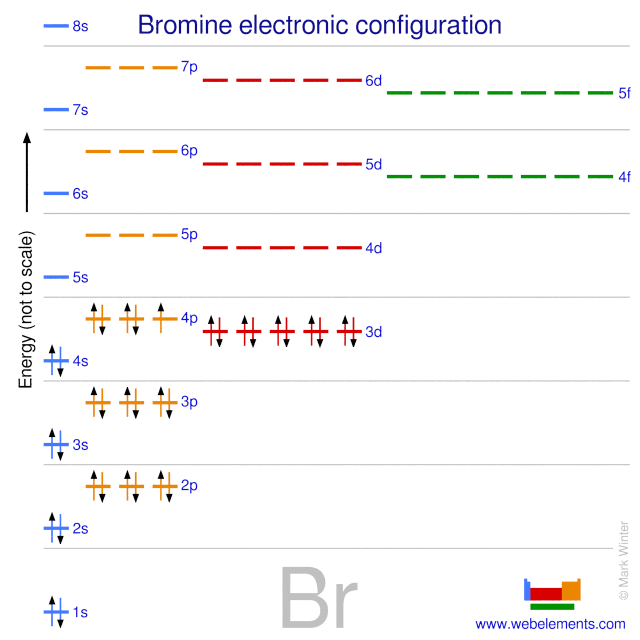
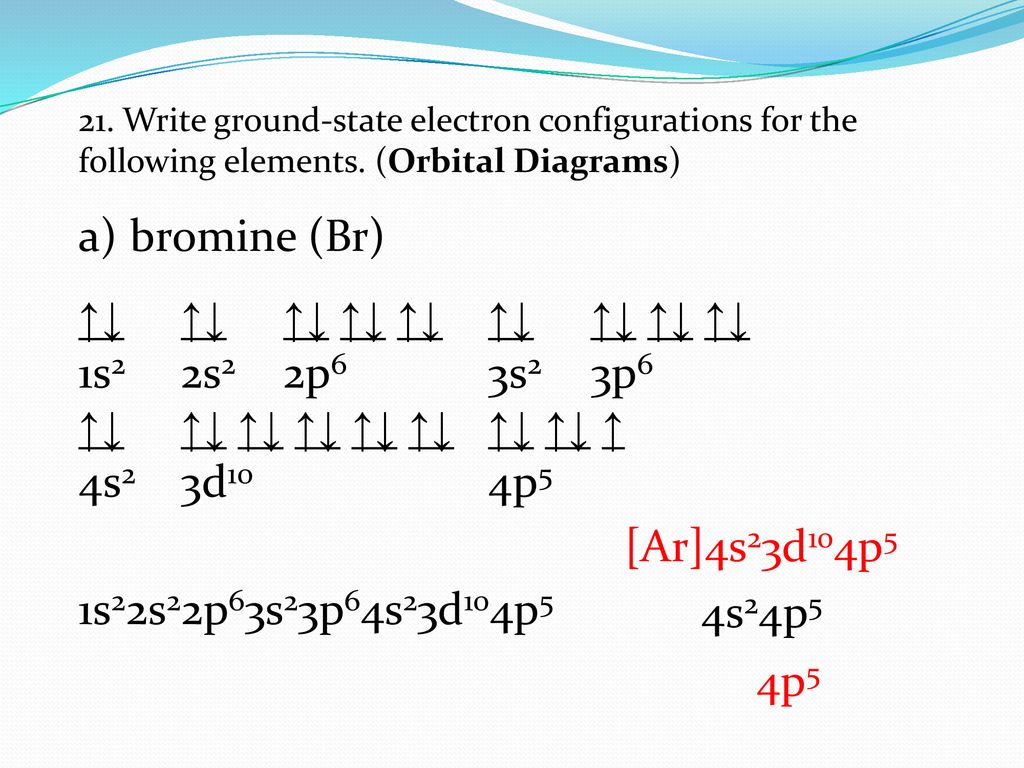
Orbital filling diagram for bromine. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Orbital-Filling Diagram for Bromine. Electrons Per Shell, 2 8 18 7. Electron Configuration, [ Ar] 4s2 3d10 4p5. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Orbital Diagram. 1s. ↿⇂. Explanation: All you need to do is work your way across the periodic table filling the orbitals ... c) Classify Cut and Cu2+ as paramagnetic or diamagnetic. 3. a) Draw the short-hand orbital filling diagram for; Question: 6.4 Electron Configurations 1. Write the complete and short-hand electron configurations for Bromine (Br). 2a) Write the short-hand electron configurations for copper (Cu). The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-17. The orbital diagram for Bromine is 2-8-18-7. Orbital filling diagram for bromine. Ne2 molecular orbital diagram periodic table q inspirationa bromine on the molecular orbital diagram c2 crystals free full orbital diagram of bromine 9 orbital diagram for bromine has 35 electrons electron configuration is 1s 2 2s 2p 6 3s 3p 4s 3d 10 4p 5. What is the last orbital fill in bromine atom.
1 answerWe will first determine the number of electrons that a neutral bromine (Br) atom has by referring to its atomic number on a periodic table. Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis ... Use the orbital filling diagrams to complete the table. Is 2s lectron Is 4s on 2s a o o gurations or ome Orbital filling elected ements Electron 3s configuration Isl C] element (answer) en on Element O Ne 2Px 2py 2pz 2. Which element has the following orbital diagram? 3. Using arrows, show how the following orbitals will fill with electrons. An orbital view of the bonding in ethyne. Aug 14, 2019 · Ne2 molecular orbital diagram. z Atomic orbital x x Y Atomic Ne2 (It'2p)4 (022p)2 131 Diamagnetic (lt2p The phase represents the sign of the wavefunction Molecular Structure We combine atoms to form molecules by considering the phase of the atomic orbitals we are using The phase represents the sign of the wavefunction We represent the ...
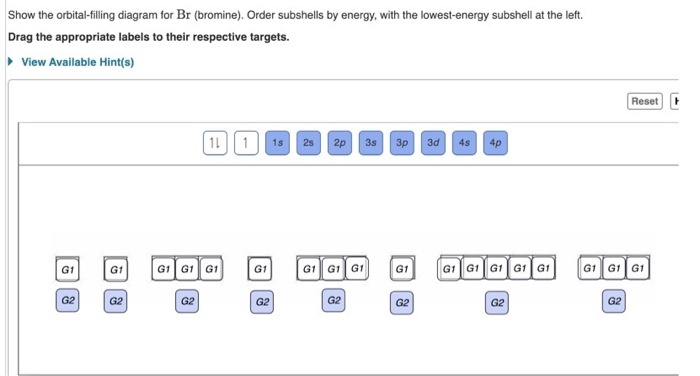
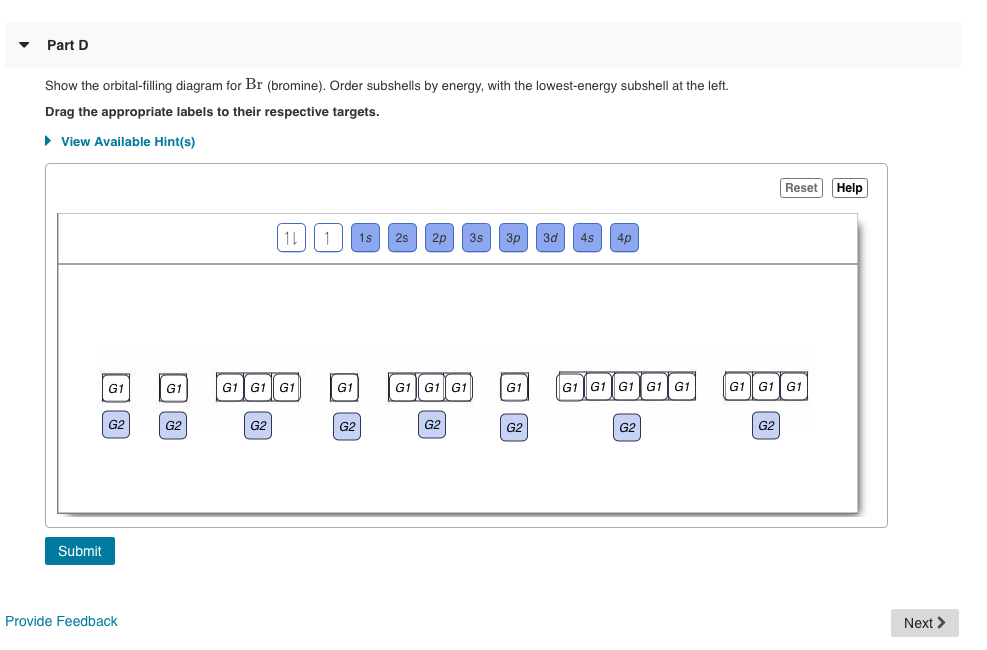
Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be (a)This diagram represents the correct filling of electrons for the nitrogen atom.The orbital diagram for Bromine is Share to: Orbital notation ... Show The Orbital-filling Diagram For Br (bromine) Answer to Show the orbital-filling diagram for (bromine). Stack the subshells in order of energy, with the lowest-energy sub shell. sodium. Is°' 35 iron. Is7 p6 3s2 3 4s 3. 3) bromine. |s* 26°p33° 3pºus30 ° 4) barium 3p. AS. 3d. 01.11.2021 · Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram of Niobium (Nb) 42: Orbital diagram of Molybdenum (Mo) 43: Orbital diagram of Technetium (Tc) 44: Orbital diagram of Ruthenium (Ru) 45: Orbital … Question: Part D Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint (s) Reset Help 1 18 25 2p 3s 3p 30 48 4p Submit Provide Feedback Next > Part B Show the orbital-filling diagram for N (nitrogen).
9 May 2021 — Bromine Orbital Diagram. You can get the idea of an atomic number of the details with the help of a periodic table if you want to know more ...

Scielo Brasil The Use Of Rich And Suter Diagrams To Explain The Electron Configurations Of Transition Elements The Use Of Rich And Suter Diagrams To Explain The Electron Configurations Of
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
1 answerRecall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Br is 35 and since it's a neutral element, this means Br ...
Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p⁶3s²3p⁴. Item 2: Part D Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the ...
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box ... "s block" elements are filling the s orbital, "p block" elements have filled the s orbital and are adding electrons to the p orbitals. We apply Hund's Rule to maximise the number of unpaired electrons in the p orbitals, that is, electrons will occupy the p orbitals singly until ...
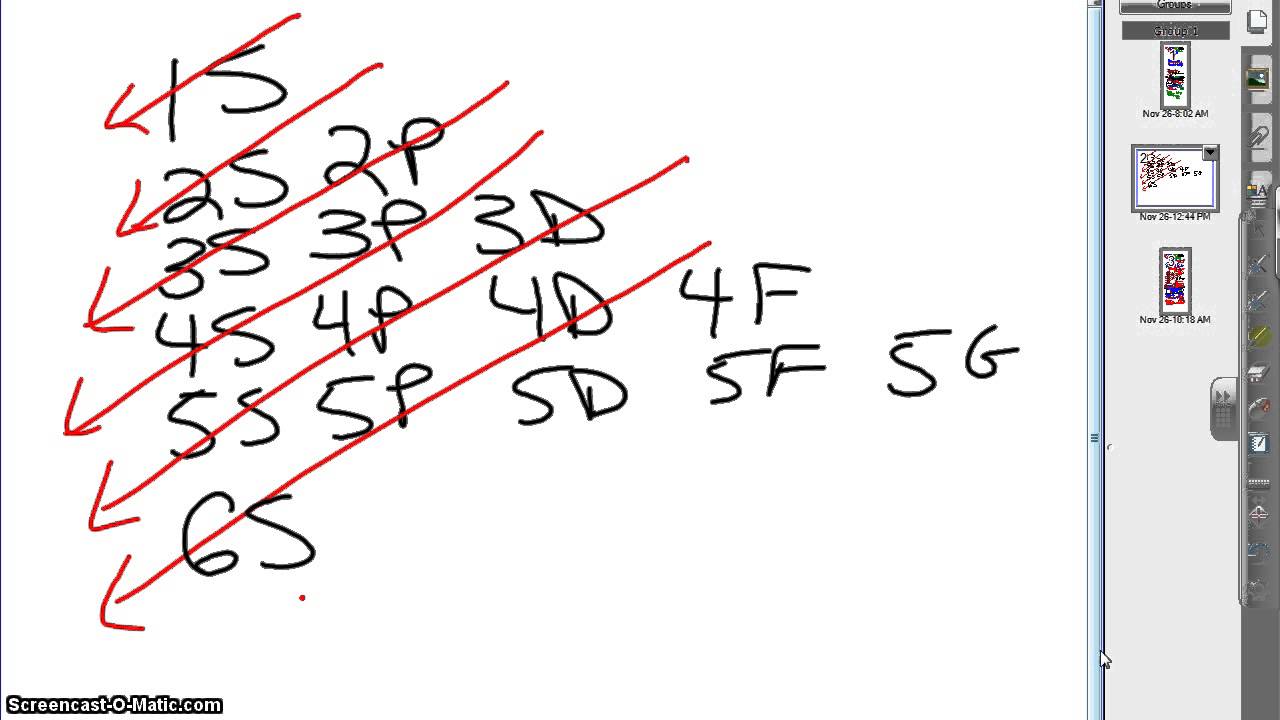
18.02.2020 · Aufbau principle. Each type of orbitals in the above diagram is colored the same and are arranged in the ascending order of the principal quantum number (n) from the top to bottom, for example, 2p, 3p, 4p, 5p, 6p…From the left to right, the orbitals are arranged according to the azimuthal quantum number (l).l = 0 corresponds to the s orbital, l = 1 corresponds to the p orbital, l = 2 ...
In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. • If you were to continue in that fashion, however, you would assign chromium and copper the following incorrect configurations. Determine the numbers of electrons which the atoms will lose and gain by applying the Octet Rule. AMU ...
Oxidation States, ±1,+5. Electrons Per Shell, 2 8 18 7. Electron Configuration, [Ar] 4s2 3d10 4p5. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Orbital Diagram.
What is a pi bond describe with the aid of a diagram What is a pi bond describe with the aid of a diagram ...

Diagram For Bromine Wiring Schematic Diagram Bromine Electron Shell Diagram For Calcium Hd Png Download Transparent Png Image Pngitem

Orbital Notations And Electron Configurations 1 2 N Principle Energy Level 2n 2 Of E In N Ppt Download
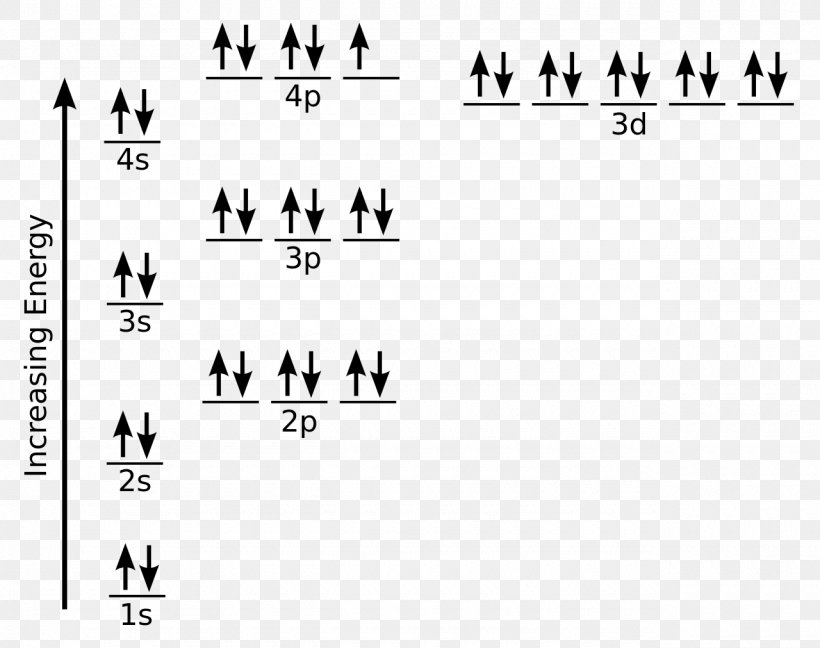
Aufbau Principle Molecular Orbital Diagram Electron Configuration Bromine Atomic Orbital Png 1280x1012px Watercolor Cartoon Flower Frame

Part A Show The Orbital Filling Diagram For N Nitrogen Order Subshells By Energy With The Lowest Energy Subshell Homeworklib

Solved Write The Complete Orbital Diagram For Each Of The Following Elements Using Boxes To Represent Orbitals And Arrows To Represent Electrons Begin Array L Text A Aluminum Z 13 Text B Phosphorus
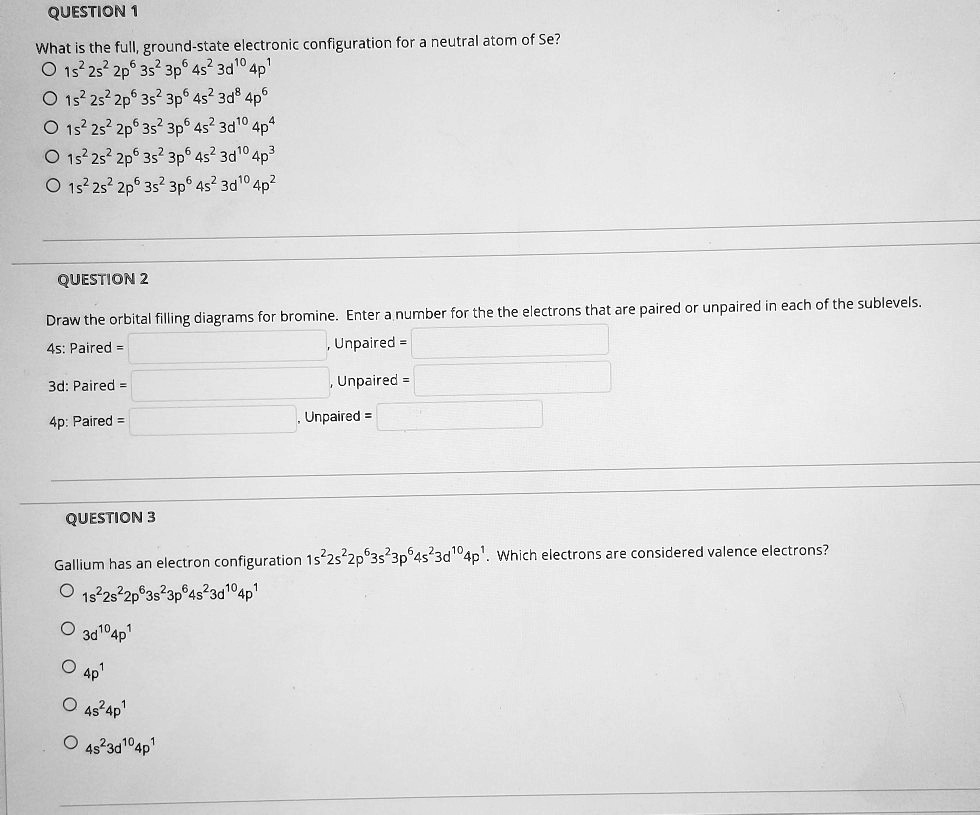
Solved Question What Is The Full Ground State Electronic Configuration For Neutral Atom Of Se 152 2s 2p6 35 3p6 4s2 3d 0 2 Ap 152 2s2 2p6 3s2 3p6 4s 3d8 4p 152 2s2
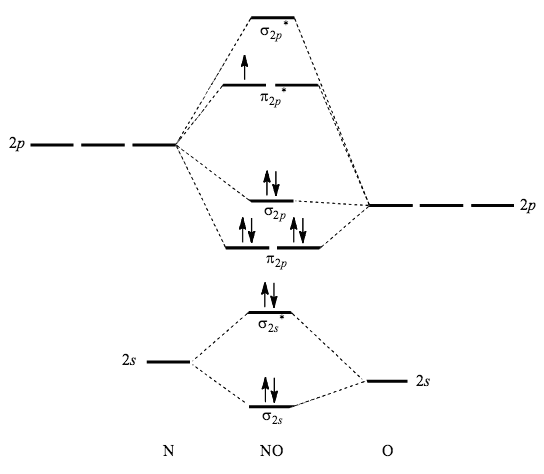
How To Draw Overlapping Of Pure Or Hybridized Orbitals For Br2 And No Explain The Need For The Orbital Of An Atom To Hybridized Based On The Lewis Structures Socratic
:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg)







/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)







0 Response to "39 orbital filling diagram for bromine"
Post a Comment