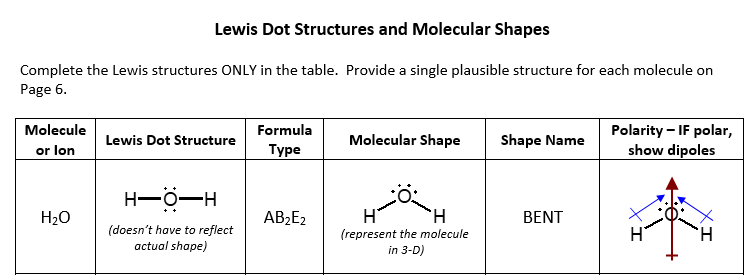
39 lewis dot diagram h2o
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc... The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist. Drawing the lewis structure for h 2 o. Bent first draw the lewis structure of water. There is an atom of oxygen in the center and two atoms of hydrogen around the central atom. It is helpful if you.
Answer (1 of 2): Firstly you need to know the number of electrons present in outermost shell of O and H atom O: 1s2 2s2 2p4 There are six electrons in outermost shell H: 1s1 there is one electron in outermost shell Write down the symbol of atom and no. of electrons in the outermost shell are r...

Lewis dot diagram h2o
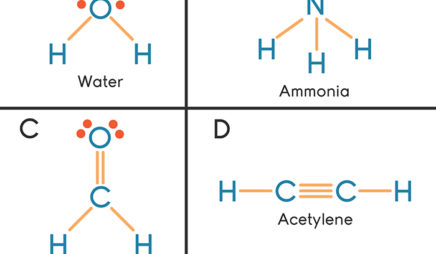
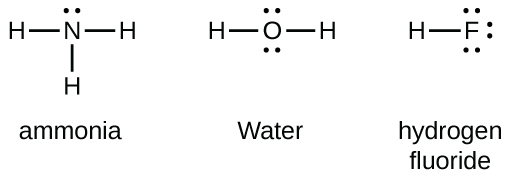
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible). Draw a Lewis dot diagram for water (H2O) b) Draw a Lewis dot diagram for methane (CH4) c) Draw a Lewis dot diagram for ammonia (NH3) Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
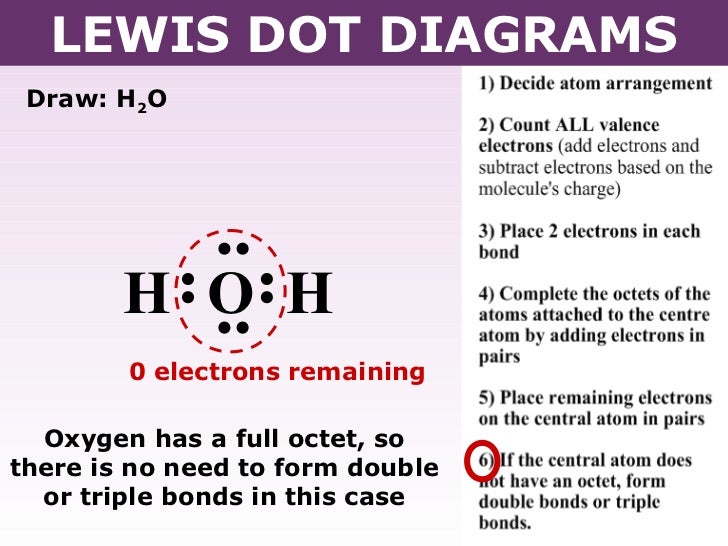
Lewis dot diagram h2o. Answer (1 of 3): Lewis sturucture is the one in which an atom or molecule is represented in the form of dots. Each dot represent an electron in an atom. For example the lewis structure of NaCl will be represented as given below. The two dots i.e the electrons between the sodium and chlorine atom ... Lewis dot diagram for h2o. Water is a transparent tasteless odorless liquid at room temperature and standard pressure. The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. While oxygens octet seems to have been filled hydrogen only has two electrons for its valence shell. Covalent bond and Lewis dot structure (H2O & CO2) Google Classroom Facebook Twitter. Email. Bonding in carbon- covalent bond. Carbon and hydrocarbons. Covalent bond . Covalent bond and Lewis dot structure (H2O & CO2) This is the currently selected item. Single and multiple covalent bonds. 1:59Hey Guys,In this video we are going to learn about the Lewis structure of H2O. It is a chemical formula for ...10 Feb 2021 · Uploaded by Geometry of Molecules
H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure.This "bent" molecular structure gives it many unique properties such as being polar.One of the most fascinating phenomena is the idea of "hydrogen bonding ... This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have. These electron pairs form electron 'clouds' that are spread out approximately This is the reason for water's bent structure. or H2O but is better represented as H2O or even H2O giving ... 2:00I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization ...1 Oct 2011 · Uploaded by kentchemistry.com Lewis Dot Diagram for Water. lewis dot structure of h2o water i quickly take you through how to draw the lewis structure of water h2o i also go over hybridization shape and bond angle water lewis structure how to draw the lewis structure a step by step explanation of how to draw the water lewis structure lewis diagrams made easy how to draw lewis dot structures
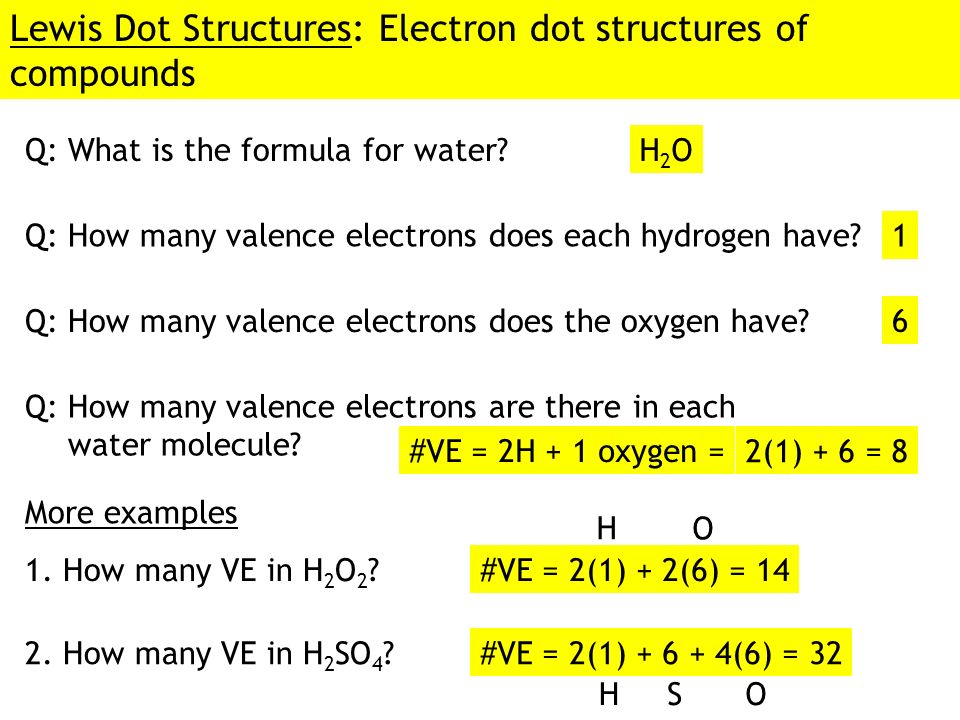
Lewis Dot Diagram for H2o. lewis dot structure of h2o water i quickly take you through how to draw the lewis structure of water h2o i also go over hybridization shape and bond angle the lewis dot structure for h2o makethebrainhappy learn what the lewis dot structure for h2o is in this post by all this is caused by the simple structure of h2o represented by the lewis dot diagram Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons. The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. In order to determine the molecular geometry for H2O observe the Lewis structure of the same. I quickly take you through how to draw the Lewis Structure of water H2O. COVID-19 is an emerging rapidly evolving situation. What is the Lewis dot structure for h2o? The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. You have eight valence electrons in your trial structure, so it has the correct number of electrons.
We have previously discussed the Lewis structures of CO2, O3, SO2, SO3 and more. Today we are going to learn about the Lewis structure of H2O molecule along with its molecular geometry and shape. Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure.
5:42The Lewis Dot Structure of Water H2O http://chemin10.com How to draw the bonds in H2O, and how the ...20 Jul 2015 · Uploaded by Chemin10
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom's nucleus and are found in principal energy levels that contain only up to a specific number of electrons.
12+ Electron Dot Structure Of H2O. Electrons of the covalent bond by two dots. In order to eject an electron from a metal, a photon of a certain minimum energy must strike the sur. There are two hydrogens that bind with 1 electron from those 6, so there are 2 electrons that are binding with hydrogen. Know how and when to incorporate double and ...
Lewis dot structures (or just Lewis structures) were developed around 1920 by pioneering chemist Gilbert Lewis, as a way of picturing chemical bonding in molecules.. We draw Lewis structures to . Discover the bonding arrangement of atoms,; Discover whether there is any degeneracy of bonding (more on that later),; Figure out whether a given group of atoms might even bond together to form a ...
7:24H2O Lewis Structure||Lewis Structure for H2O (Water)||Lewis Dot Structure for Water(H2O)||How many ...29 Oct 2019 · Uploaded by Chemistry 360
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
1 Answer. Ernest Z. Jul 15, 2014. You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom. The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.
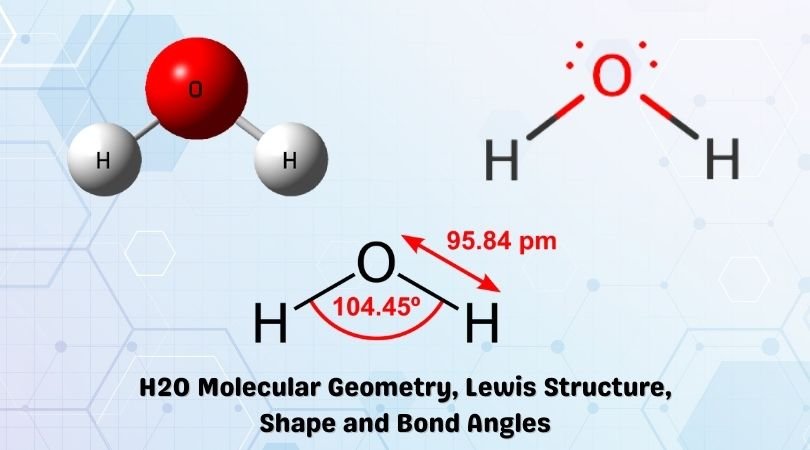
The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Moreover, these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule. It is the reason why the bond angle that should have ...
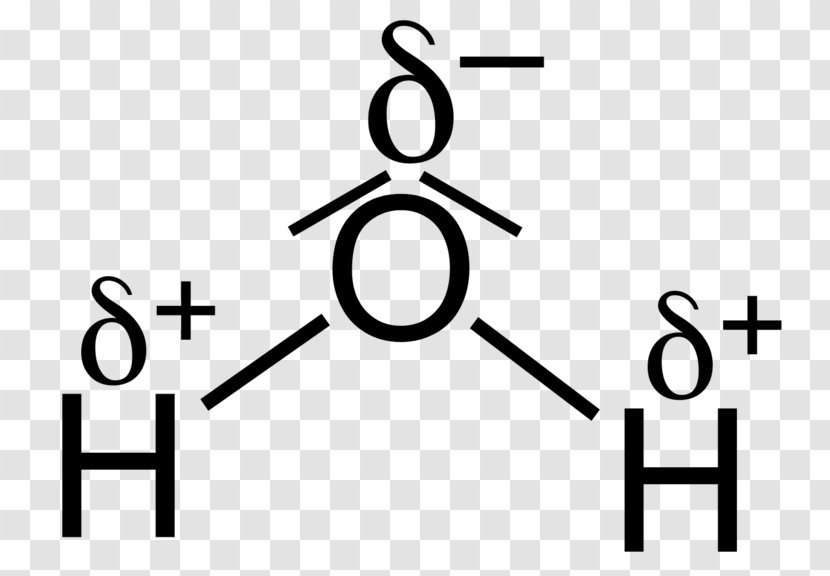
Alternate view of lewis dot structure of water: This arrangement of shared electrons between O and H results in the oxygen atom having an octet of electrons, and each H atom having two valence electrons. What is the structure of bf3? The geometry of molecule of BF3 is 'Trigonal Planar. ' With the reference of Chemistry, 'Trigonal Planar' is a ...
H 2 O Lewis Structure Video. Video Transcript: Here, we're going to do a dot structure for water, H2O. Let's write that down: H2O. What we want to find out first is how many valence electrons does water have. I'm counting all the outer shell electrons. I'll need my periodic table.
Lewis dot structure of H 2 CO. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. H:1x2=2 C:4 O Total= May 03, · May 6, - Uploaded by Wayne Breslyn. For the CH2O Lewis structure, calculate the total number of valence electrons for the CH2O molecule.
The diagram opposite shows the lewis structure for the water molecule, h2o, and the ethene molecule, c2h4. 10+ C2H6O Lewis Structure. • for main group elements, the number of valence e − is equal to the group number. The structure on the right is the lewis electron structure, or lewis structure, for h2o.
Lewis Structure of H 2 O (Water) - Drawing Steps. Lewis structure of water molecule contains two single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Each step of drawing lewis structure of H 2 O are explained in this tutorial.
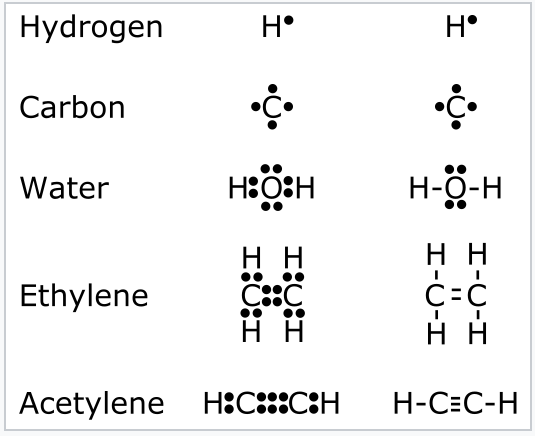
1 2 Valence Bond Theory Lewis Dot Structures The Octet Rule Formal Charge Resonance And The Isoelectronic Principle Chemistry Libretexts
Therefore, corresponding to the question, we can that the correct Lewis dot structure of water molecules is number 3. So, Option C is the correct answer. Note: (1) Lewis structures also known as Lewis dot structures or electron dot structures are diagrams that represent the valence electrons of an atom within a molecule.
Draw a Lewis dot diagram for water (H2O) b) Draw a Lewis dot diagram for methane (CH4) c) Draw a Lewis dot diagram for ammonia (NH3) Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
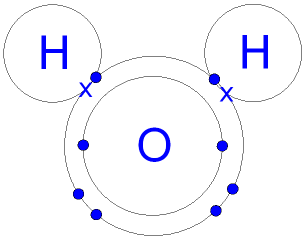
Gcse Chemistry Covalent Bonding In A Water Molecule What Is The Structure Of A Water Molecule Gcse Science

H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Molecular Geometry Molecular Shapes Intermolecular Force

Two Students Made The Lewis Dot Diagrams Of H2o The Diagrams Are As Shown Which Student Drew The Brainly Com


:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)













/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)


0 Response to "39 lewis dot diagram h2o"
Post a Comment