39 lewis diagram for bf3
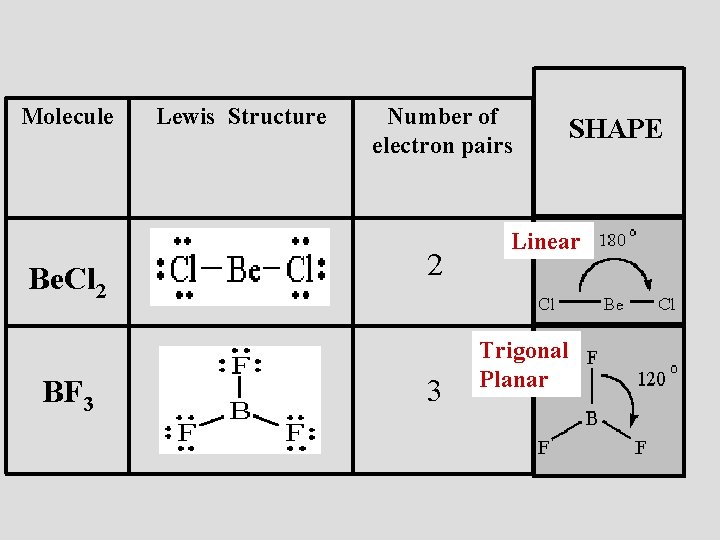
BF3 is nonpolar. When the difference in electronegativity between the two atoms is less than 0.5, it is majority nonpolar. I hope this article made sense to you and helped you to understand BF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. Step 1: Use lewis structure guidelines to draw the lewis structure of BF 3. Step2: Apply VSEPR notation, A X E A=Number of central atoms X=Number of surrounding atoms E= Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 3 E 0. Step 3: Use VSEPR table to find the shape. AX 3 has trigonal planar shape.
BrF3 has a T-shaped or Trigonal Bipyramidal molecular geometry with a bond angle of 862 which is somewhat less than the typical 90. Its like peripheral atoms all in one plane as all three of them are similar with the 120 bond angles on each that makes them an equilateral triangle. There are a total of 28 valence electrons for the BrF 3 Lewis ...

Lewis diagram for bf3
K C Q Molecule Lewis Structure Polar or non polar? H2O Br2 CH4 NH3 BF3 CH3Br Polarity Flow Chart Handout. Polarity. What’s happening inside covalent molecules like O 2 or H 2? Electrons are shared equally. Molecules become POLARwhen electrons are not shared equally HF is covalent Show activity on this post. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding. 2p (along the bond axis) for F has an ... Mit Wasser reagiert es unter Zersetzung zu Borsäure und Flusssäure. Im Gegensatz zu den anderen Bortrihalogeniden findet keine direkte Hydrolyse statt, sondern eine Abfolge mehrerer Reaktionen. Dabei bildet sich aufgrund der starken BF3-Bindung zunächst ein Lewis-Säure-Base-Addukt.
Lewis diagram for bf3. October 7, 2015 - $\ce{BF3}$ does back bonding with fluorine and still accepts a pair of electron and is considered lewis acid why? BF3, boron trifluoride, is a tricky molecule to draw because Boron is an exception to the octet rule.It does not need eight electrons in its outer shell, although it can hold eight just like most other non-metals.. The Lewis Structure of BF3, boron trifluoride, has one boron atom in the centre, and three fluorine atoms surrounding it. Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material.Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing. Thus, the structure of BF3, with single bonds, and 6 valence electrons around the central boron is the most likely structure
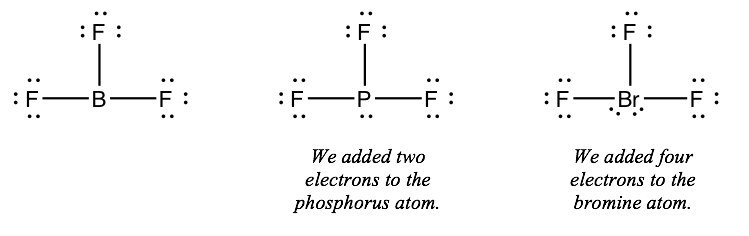
What is the Lewis Structure for BF3, the steric number, the electron pair geometry, the molecular geometry, and how many double bonds does it have? close. Start your trial now! First week only $4.99! arrow_forward. Question. NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. NF3 has a molar mass of around 71.002 g/mol and a density of 3.003 kg/m3. Lewis Dot Structure For Boron Trifluoride, BF3 Lewis Structure: How to Draw the Lewis Structure fo, BF3 Lewis Structure, Molecular Geometry, Hybridization, Lewis Diagram For Bf3 General Wiring Diagram, Boron trifluoride Alchetron, The Free Social Encyclopedia The BBr3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the BBr3 molecule. The geometry of the BBr3 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the BBr3 geometrical shape in which the ...
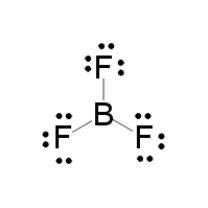
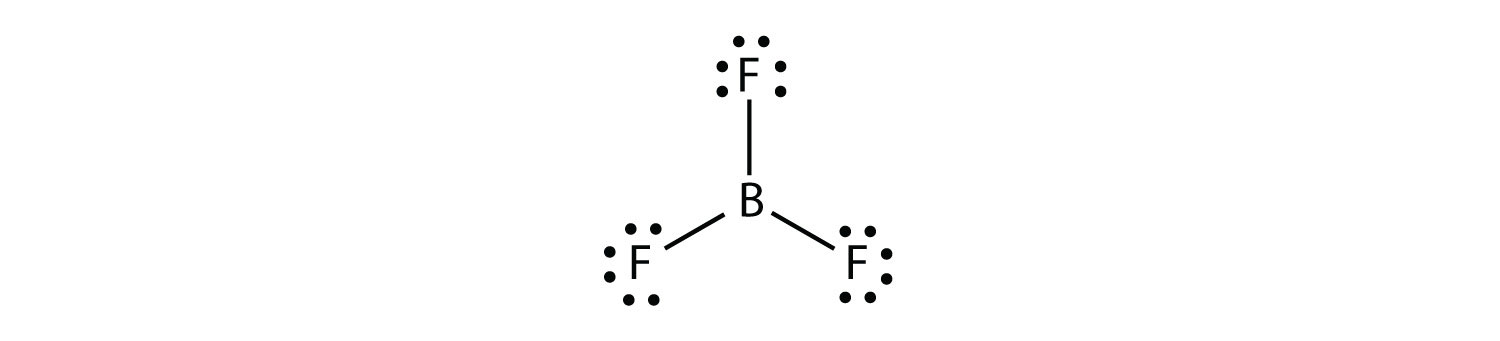
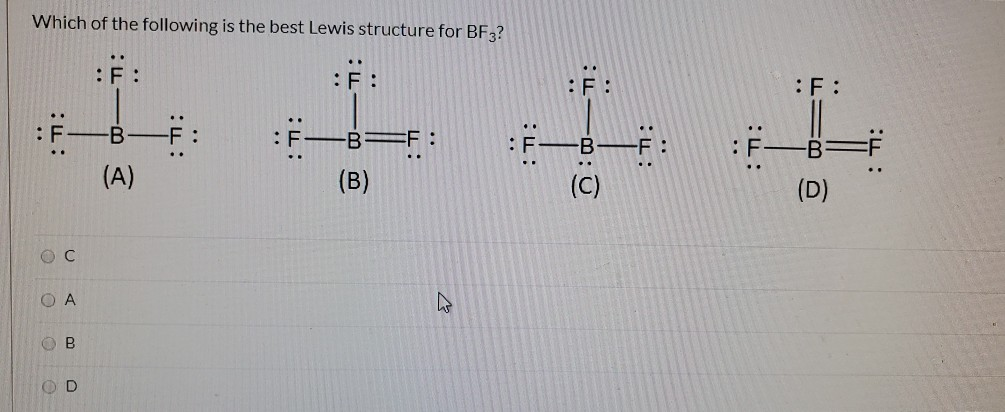
Das Lewis-Säure-Base-Konzept ist eine Definition der Begriffe Säure und Base, die unabhängig von Protonen im chemischen Sinn ist. Sie wurde 1923 von Gilbert Newton Lewis eingeführt. ... Eine Lewis-Säure ist ein elektrophiler Elektronenpaarakzeptor, kann also Elektronenpaare anlagern. Lewis Dot Structure For Boron Trifluoride Bf3, Violations of the Octet Rule Chemistry LibreTexts, Lewis Diagram For Bf3 General Wiring Diagram, BF3 Lewis and 3 D Structures Dr Sundin UW Platteville, Lewis Dot Structure of BCl3 (Boron TriChloride) YouTube There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure. Boron trifluoride only has six valence electrons and is one of the relatively rare second period covalent molecules that disobeys the octet rule. There are three bonded groups and so no lone pairs. Six electrons implies three electron pairs and therefore a trigonal geometry · A VSEPR tutorial ...
This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds. ... The geometry of a molecule of BF3 is trigonal planar. Its D3h symmetry conforms with the prediction of VSEPR theory.

Draw The Lewis Structure For Bf3 How Many Bonds Are Around The Central Atom And What Is The Shape Of This Molecule Study Com
By analyzing the Lewis structure of SO2, we can see that the SO2 is asymmetrical because it contains a region with different sharing. The molecular geometry of SO2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of Oxygen have more of it. So, the conclusion is, SO2 is a Polar molecule.
February 27, 2009 - Lewis-Basen und -Säuren BF3 Allgemeine Chemie
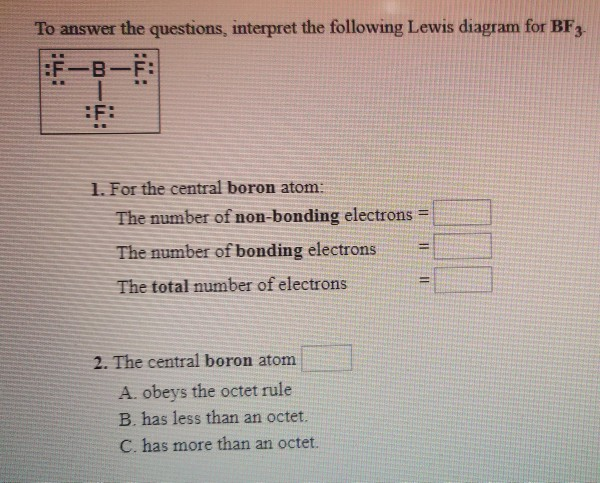
Electron Dot Diagram for BF3 · Boron trifluoride is electron deficient · The octet rule does not apply · Boron is the central atom · Actual # valence electrons = (3 x 7) + 3 = 24 · Maximum # valence electrons = 4 x 8 = 32 · Difference = 32 – 24 = 8; 4 bonds should be present, but only ...
Chemistry questions and answers. The Lewis diagram for BF_3 is: The electron-pair geometry around the B atom in BF_3 is There are lone pair (s) around the central atom, so the geometry of BF_3 is The Lewis diagram for BeF_2 is: The electron-pair geometry around the Be atom in BeF_2 is There are lone pairs around the central atom. so the ...
Draw Bf 3 And Assign A Point Group How Many Degrees Of Vibrational Freedom Does The Molecule Have Socratic
BF3 Lewis Structure, Molecular Geometry, and Hybridization Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature.
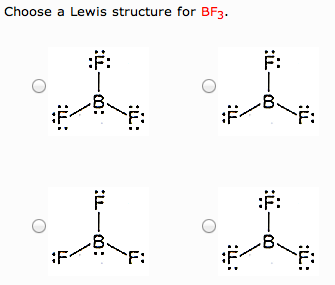
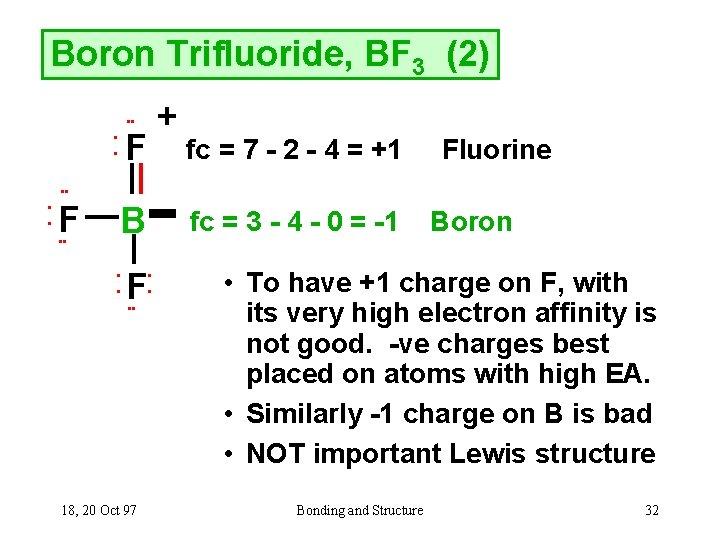
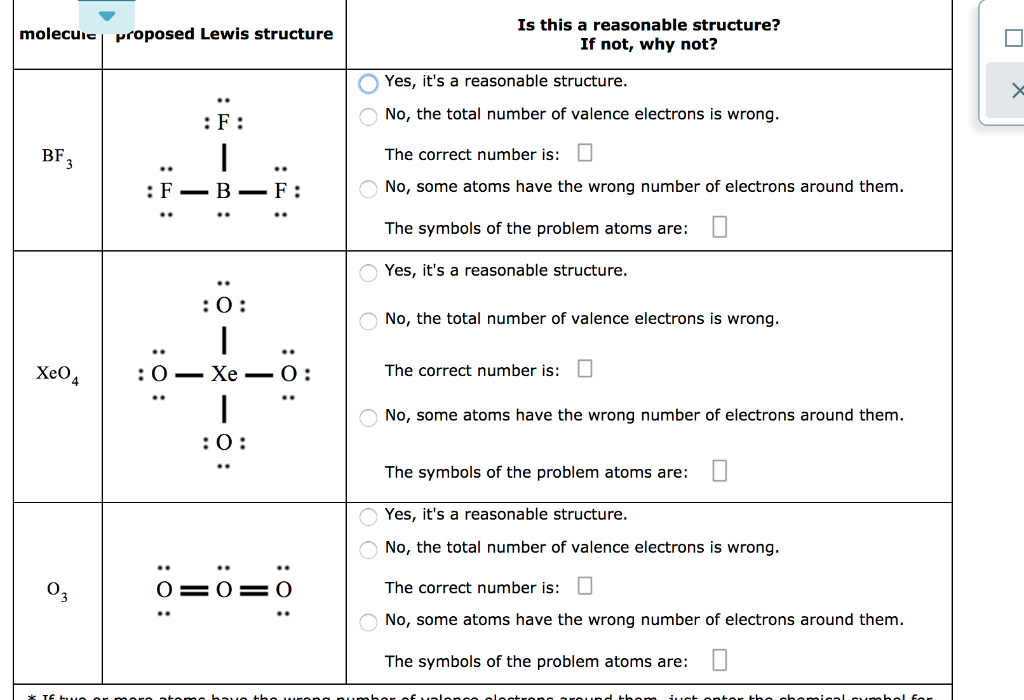
Its formula is = [no. of valence electrons on atom] - [non-bonded electrons + number of bonds]. or a simple one {if no lone pair are there} = 1/2 no. of bonds.)on each atom. Note:Boron disobeys octet rule in Lewis structure. Its a property of it about which we cannot do anything. (in fig. 1). We must examine the formal charges of this structure.
Made with Explain Everything
Drawing the Lewis Structure for BF 3. Viewing Notes: The BF 3 Lewis structure is similar to BCl 3 and BBr 3 since Cl and Br are in Group 7 and have 7 valence electrons.; Boron (B) doesn't need 8 valence electrons to have an octet (Boron often only needs 6). If you're not sure you have the best Lewis structure for BF 3 you can calculate the formal charges. You'll find the B in BF 3 only has 6 ...
When NH3 contributes its lone pair of electrons to BF3, it serves as a Lewis base in this diagram. When BF3 absorbs the lone pair of electrons that NH3 donates, it becomes a Lewis acid. This reaction fills BF3's vacant 2p-orbital, making boron sp3 hybridize where it was previously sp2 hybridized (as BF3).
BF3 Lewis Structure. If you want to know about the lewis structure of BF3, you need to calculate the valence electrons for the BF3 molecule. Before calculating the valence electrons, make sure you know that there are a total of 24 valence electrons for the lewis structure of BF3.
June 3, 2018 - Answer (1 of 10): BF3 is electron deficient molecule and has an empty p-orbital, so it can accept a pair of electrons, making it a Lewis acid. A Lewis acid can accept a pair of electrons from atom of same or different molecules (known as Lewis base). Lewis acid strength of BF3 decrease due to 2p...
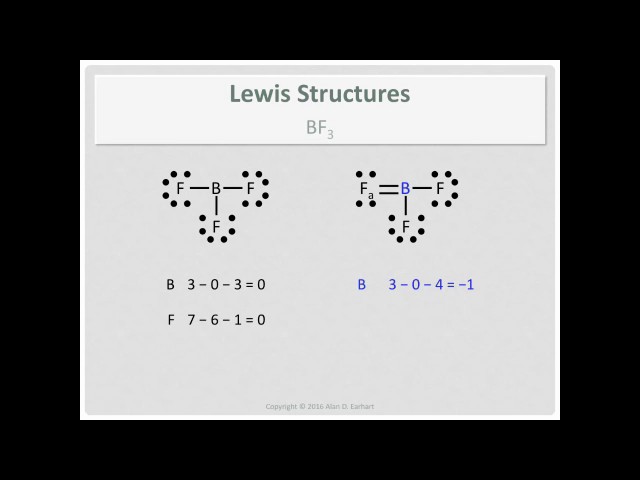
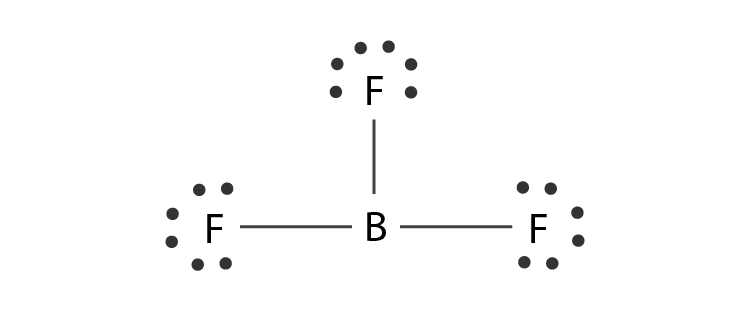
In the BF3 Lewis structure diagram, the boron atom can be the center atom of the molecule. As a result, central boron in the BF3 Lewis structure, with all three fluorine arranged in the trigonal planar geometry. Add valence electrons around the fluorine atom, as given in the figure. Step-3: Lewis dot Structure for BF3 generated from step-1 and step-2
If you look at the Lewis Structure for BF3 it appears to be a symmetrical molecule. However, to determine if BF3 is polar we consider the molecular geometry. A polar molecule results from an unequal/unsymmetrical sharing of valence electrons. For Boron trifluoride the molecule is symmetrical and therefore it is a nonpolar molecule.
Lewis Structure of Boron Trifluoride (BF. 3. ) Boron trifluoride contains one boron atom and three fluorine atoms. Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of ...
The boron atom in boron trifluoride, BF3, has only six electrons in its valence shell. Being short of the preferred octet, BF3 is a very good Lewis acid and reacts with many Lewis bases; a fluoride ion is the Lewis base in this reaction, donating one of its lone pairs:
Hey Guys,Today we are going to look at the Lewis Structure of BrF3 which is a chemical formula for Bromine Trifluoride. The molecule comprises one Bromine at...
Mit Wasser reagiert es unter Zersetzung zu Borsäure und Flusssäure. Im Gegensatz zu den anderen Bortrihalogeniden findet keine direkte Hydrolyse statt, sondern eine Abfolge mehrerer Reaktionen. Dabei bildet sich aufgrund der starken BF3-Bindung zunächst ein Lewis-Säure-Base-Addukt.
A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures
Drawing the Lewis Structure for BF 3. Video: Drawing the Lewis Structure for BF 3. For the BF 3 Lewis structure, calculate the total number of valence electrons for the BF 3 molecule. There are a total of 24 valence electrons for the BF 3 Lewis structure. After determining how many valence electrons there are in BF 3, place them around the central atom to complete the octets.
August 15, 2020 - However, boron has an electronegativity ... BH3 with Lewis theory. One of the things that may account for BH3's incomplete octet is that it is commonly a transitory species, formed temporarily in reactions that involve multiple steps. Let's take a look at another incomplete octet situation dealing with boron, BF3 (Boron ...
Drawing the Lewis Structure for BrF 3. Video: Drawing the Lewis Structure for BrF 3. For the BrF 3 Lewis structure, calculate the total number of valence electrons for the BrF 3 molecule. There are a total of 28 valence electrons for the BrF 3 Lewis structure. After determining how many valence electrons there are in BrF 3, place them around the central atom to complete the octets.
A step-by-step explanation of how to draw the BF3 Lewis Dot Structure (Boron Trifluoride).For the BF3 Lewis structure, calculate the total number of valence ...
BF3, boron trifluoride, is a tricky molecule to draw because Boron is an exception to the octet rule.It does not need eight electrons in its outer shell, although it can hold eight just like most other non-metals.. The Lewis Structure of BF3, boron trifluoride, has one boron atom in the centre, and three fluorine atoms surrounding it.
Alternatively a dot method can be used to draw the lewis structure of BF3. Calculate the total valence electrons in BF3 molecule. B=3 F=7x3=21 Total=24 Put Boron in the center and three fluorine atoms on the sides. Put a pair of electrons connecting the side atom with central atom.Pur remaining ...
March 28, 2017 - Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.

Molecular Shapes If You Were To Draw The Lewis Structure For Carbon Tetrachloride Based On What You Have Already Taken In This Class You May Come Up With Ppt Download
What is Lewis structure of bf3? There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.
Mit Wasser reagiert es unter Zersetzung zu Borsäure und Flusssäure. Im Gegensatz zu den anderen Bortrihalogeniden findet keine direkte Hydrolyse statt, sondern eine Abfolge mehrerer Reaktionen. Dabei bildet sich aufgrund der starken BF3-Bindung zunächst ein Lewis-Säure-Base-Addukt.
Show activity on this post. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding. 2p (along the bond axis) for F has an ...
K C Q Molecule Lewis Structure Polar or non polar? H2O Br2 CH4 NH3 BF3 CH3Br Polarity Flow Chart Handout. Polarity. What’s happening inside covalent molecules like O 2 or H 2? Electrons are shared equally. Molecules become POLARwhen electrons are not shared equally HF is covalent



















0 Response to "39 lewis diagram for bf3"
Post a Comment