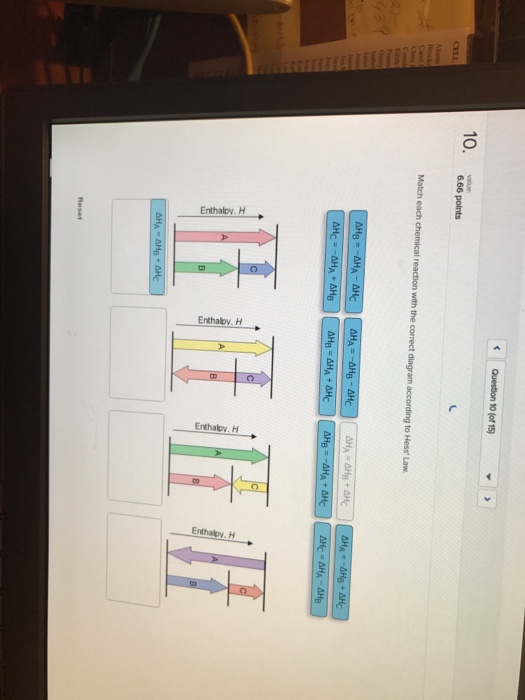
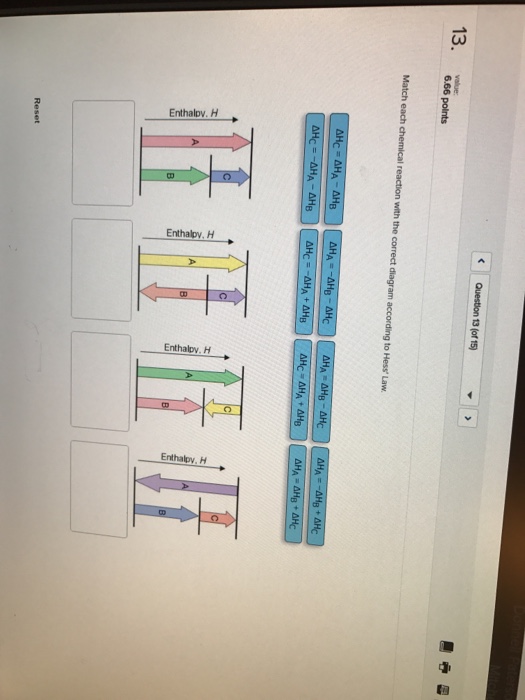
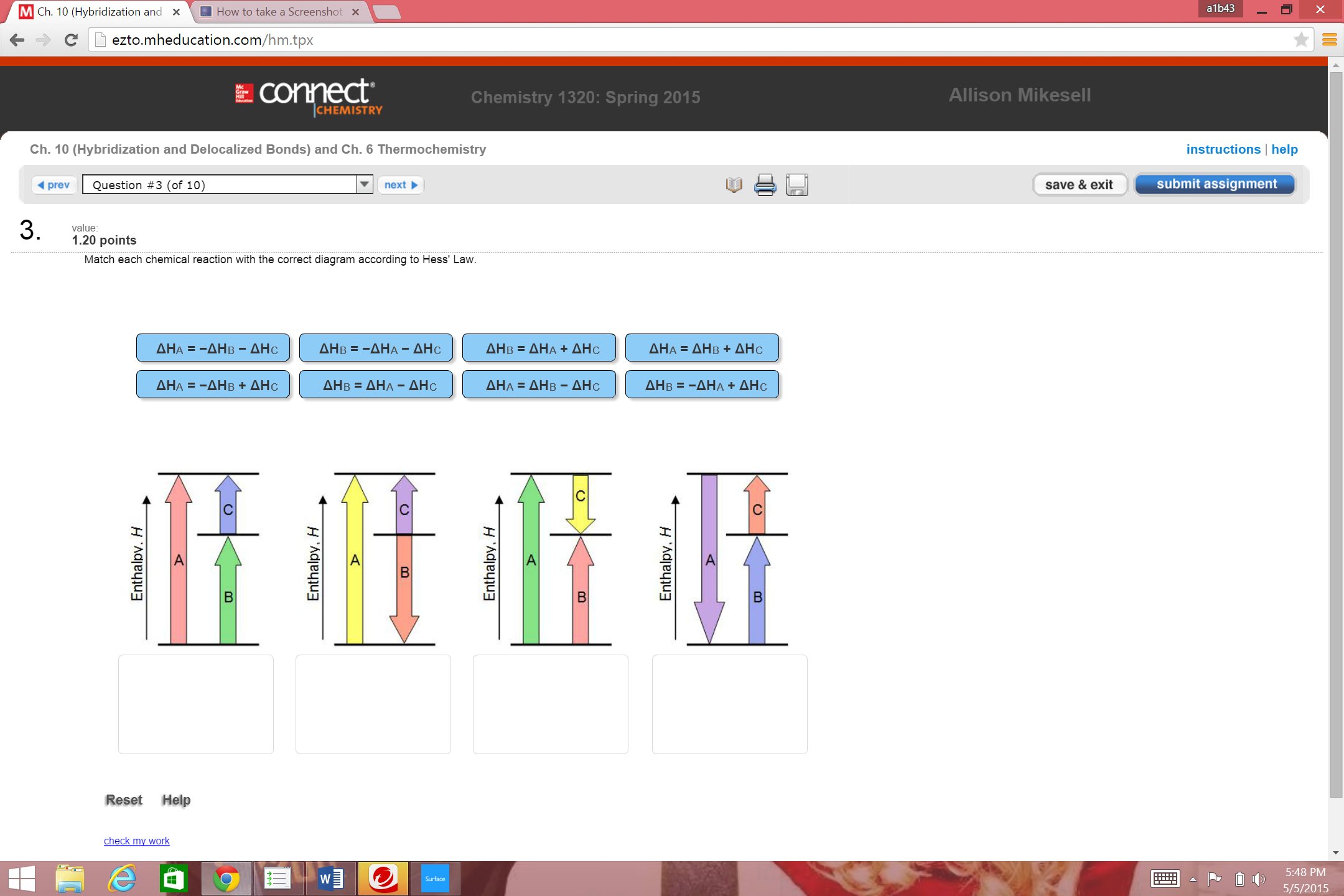
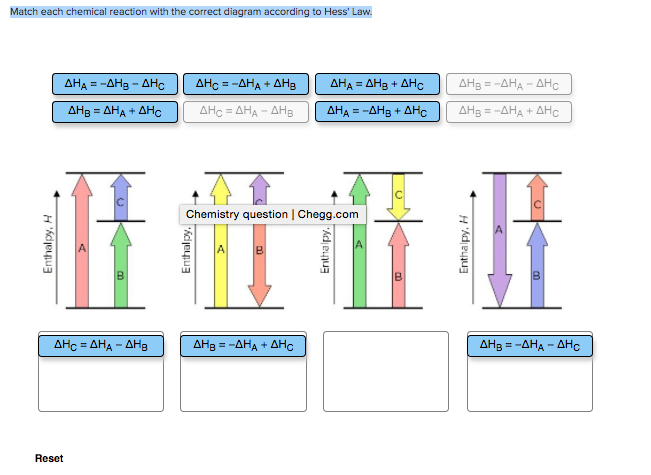
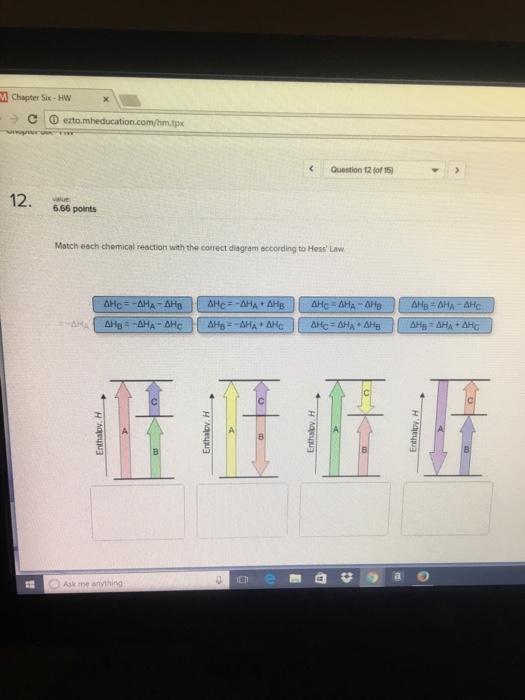
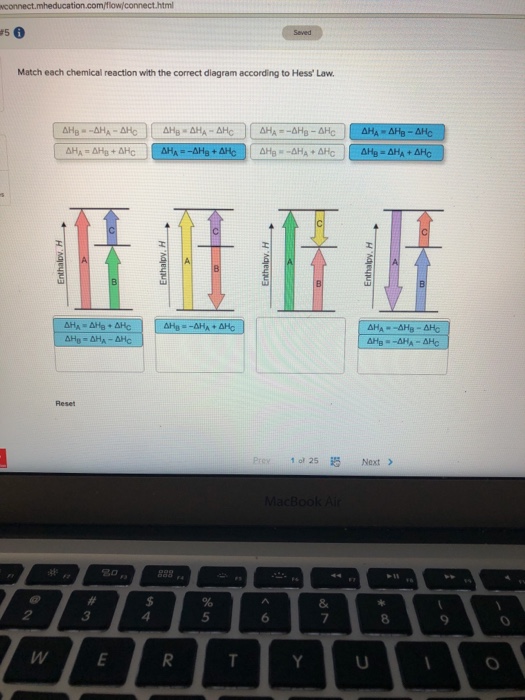
38 match each chemical reaction with the correct diagram according to hess' law.
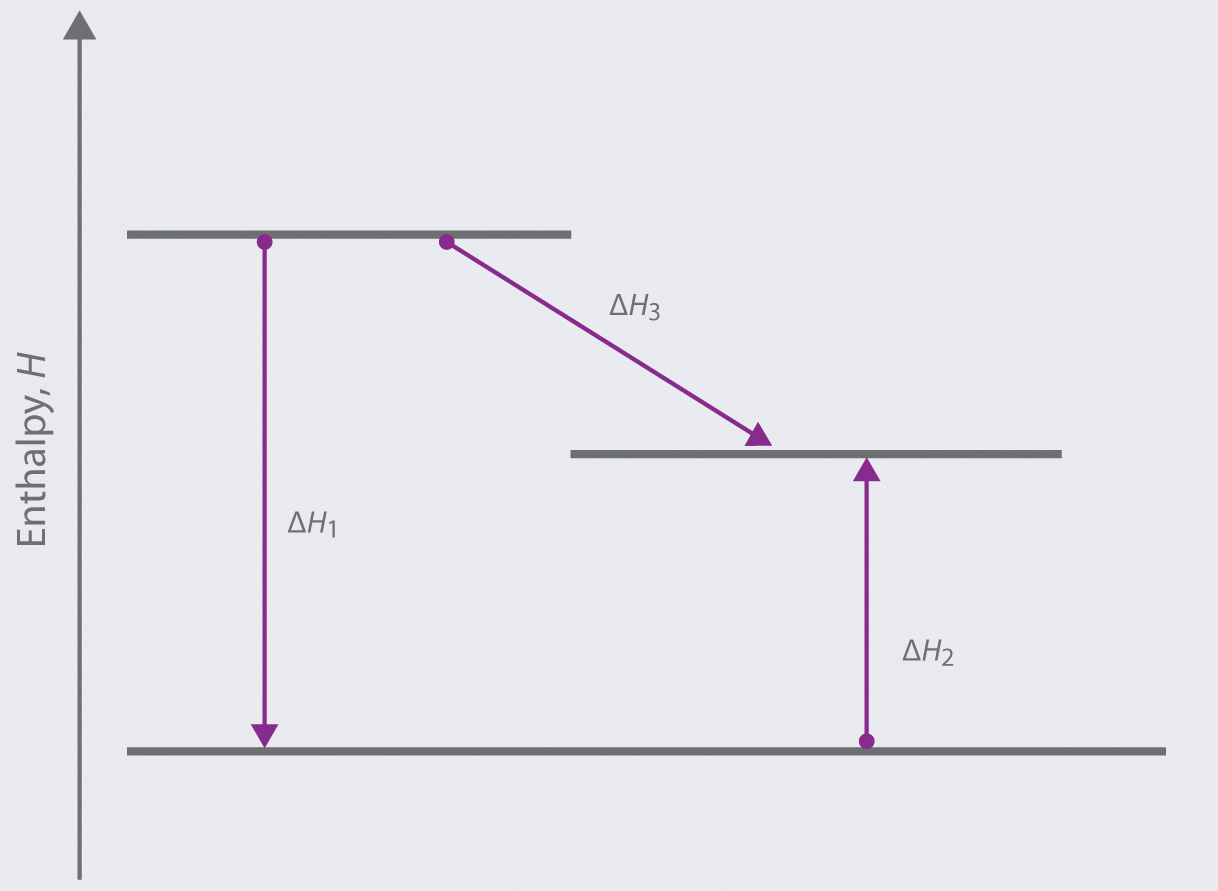
To calculate the standard enthalpy of formation of H2O(l) according to Hess's law, add the individual reactions to get the overall equation, which is H2(g)+12O2(g)→H2O(l)H2(g)+12O2(g)→H2O(l). This requires reversing equations 1 and 2 and halving equation 2 before adding the ΔH° values to determine the net enthalpy. 19.02.2020 · From these data the enthalpy of combustion of graphite to CO can be calculated by applying Hess’s law. The reactions involved in this process can be expressed as follows: According to Hess law, ∆H 1 = ∆H 2 + ∆H 3-393.5 kJ = X – 283.5 kJ X = – 110.5 kJ. Question 7. Calcu late the lattice energy of sodium chloride using Born-Haber ...
To use Hess's law and thermochemical cycles to calculate enthalpy changes of ... than a single match, even though the chemical reaction—the combustion of ...

Match each chemical reaction with the correct diagram according to hess' law.
19.11.2021 · Gas laws summative assessment answer key. Sample answers are shown. 9 9. the gas law that states that the rate of effusion is inversely…. Jul 20, … 22.11.2021 · Chemistry unit 6 reaction equations worksheet 1 part 2 answer key. Chemistry unit 6 reaction equations worksheet 1 part 2 answer key. Chemistry unit 6 … identify the bonds that must be broken and formed in the course of a chemical reaction and calculate the heat of reaction using a table of bond energies. Hess’ Law: use Hess’ Law of the additivity of heats of reaction to calculate `Delta H` for unknown reactions by …
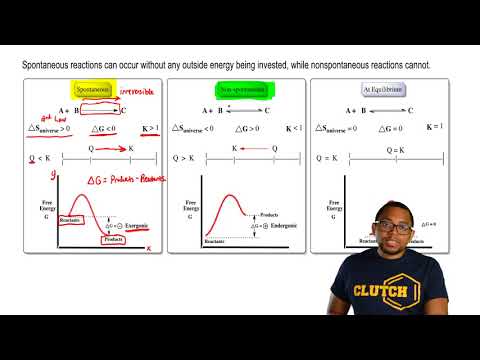
Match each chemical reaction with the correct diagram according to hess' law.. Solution for Match each chemical reaction with the correct diagram according to Hess' Law. AHA = AH8 – AHc ΔΗΒ- ΔΗΑ- ΔHC AH3 = AHA + AHc ΔΗΒΔΗΑ AH3 = -AHA + ... Problem: Match each chemical reaction with the correct diagram according to Hess' LawΔHC = -ΔHA + ΔHB ΔHB = ΔHA + ΔHCΔHA = ΔHB - ΔHCΔHB = -ΔHA - ΔHC ΔHA ...1 answer · Top answer: Assuming:Upward → positive (+) value of ΔHDownward → negative (-) value of ΔHΔHA = ΔHB + ΔHC ΔHA = ΔHB + ΔHC-ΔHC - ΔHCΔHB = ΔHA - ΔHC[readmore] ... Gibbs Free Energy, Spontaneity and Entropy. According to the 2 nd Law of Thermodynamics, the entropy of the universe always increases for a spontaneous process. This is illustrated by the equation: Second Law of Thermodynamics. Gibbs Free Energy is a thermodynamic property that can be used in determining the direction of a spontaneous process at either constant temperature (isothermal) or ... May 8, 2018 — Match each chemical reaction with the correct diagram according to hess law. Different reaction pathways can be represented on the same ...
Rebecca W. Chemistry 101. 3 months ago. Match each chemical reaction with the correct diagram according to Hess' Law: AHB ~AHA + AHc AHB = ~AHA AHc AHA =- ...4 answers · Top answer: a Z. We know that the component will must be from its constituent in their most favorable elemental ... The phrase “chemical reaction” conjures up images of explosions, bubbling gases, flames, and smoke. So many chemical reactions have visible results because energy is being transferred from one form to another—the realm of thermodynamics. Match each chemical reaction with the correct diagram according to Hess' Law... On Exam #3 draw on flash card. (#7). Question: Match each chemical reaction with the correct diagram according to Hess' Law. This problem has been solved! See the answer ...
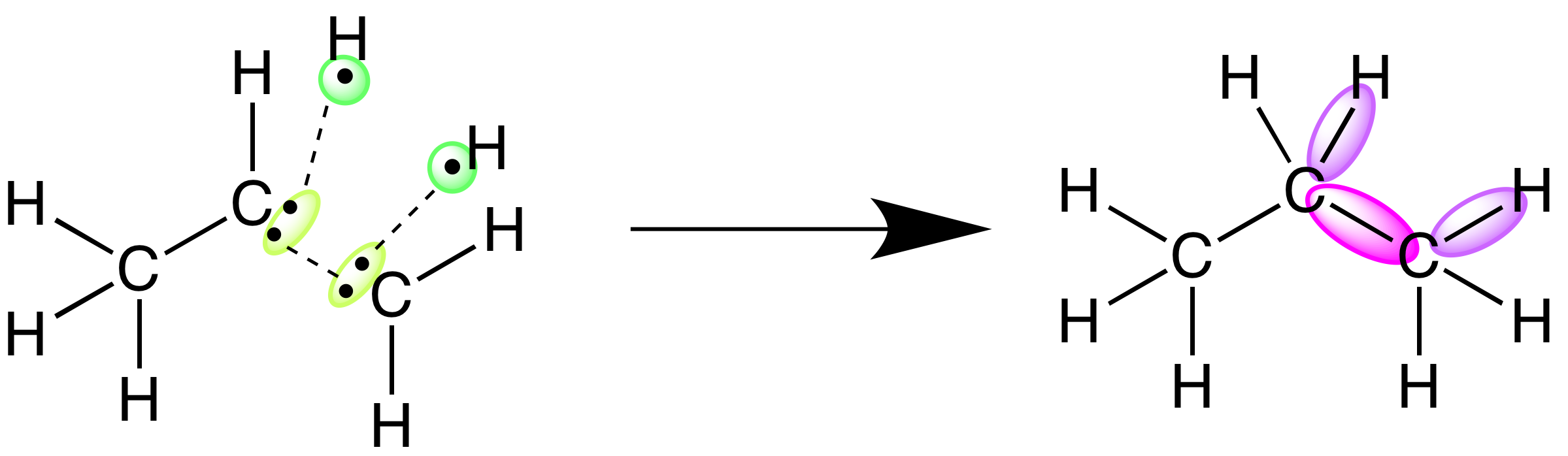
enthalpy is the heat gained or lost at a constant pressure. It is a state function because the change depends only on the initial and final states of the system, not how the change occurred. This relates to Hess's Law because a reaction can undergo a lot of steps, but the overall enthalpy change equals the sum of the individual steps. Three forms of energy that chemical energy may transform into. first law of thermodynamics, Laws of thermodynamics, Physics 06 08 the 1st law of thermodynamics and simple, Application of the first law of thermodynamics to the, First law of thermodynamics exercises, Thermodynamics homework 4, In each case does the gas do work or is work done on the. identify the bonds that must be broken and formed in the course of a chemical reaction and calculate the heat of reaction using a table of bond energies. Hess’ Law: use Hess’ Law of the additivity of heats of reaction to calculate `Delta H` for unknown reactions by summing thermochemical equations. Kinetics. Reaction rate: The First Law of Thermodynamics: The total energy content of the universe is ... Chemical Reactions: Energy is absorbed to break chemical bonds & energy is.
Nov 24, 2021 · From Hess's law we know that we can add the energies of each step in the cycle to determine the energy of the overall process. 11. Solar energy is a product of the exothermic nuclear fusion of hydrogen atoms in the star’s core. does not absorb any energy C. a) removal of electrons and hydrogens. edu and netfiles.
identify the bonds that must be broken and formed in the course of a chemical reaction and calculate the heat of reaction using a table of bond energies. Hess’ Law: use Hess’ Law of the additivity of heats of reaction to calculate `Delta H` for unknown reactions by …
22.11.2021 · Chemistry unit 6 reaction equations worksheet 1 part 2 answer key. Chemistry unit 6 reaction equations worksheet 1 part 2 answer key. Chemistry unit 6 …
19.11.2021 · Gas laws summative assessment answer key. Sample answers are shown. 9 9. the gas law that states that the rate of effusion is inversely…. Jul 20, …











0 Response to "38 match each chemical reaction with the correct diagram according to hess' law."
Post a Comment